Bent Chemistry Shape: Understanding Molecular Geometry and Bonding

Understanding the bent chemistry shape is crucial for grasping molecular geometry and chemical bonding. This shape, characterized by a central atom bonded to two other atoms with a lone pair, plays a significant role in determining a molecule's properties. Whether you're a student, researcher, or simply curious about chemistry, this guide will help you master the concept of bent molecular geometry and its implications in various fields, including organic chemistry, inorganic chemistry, and material science.
What is Bent Chemistry Shape?

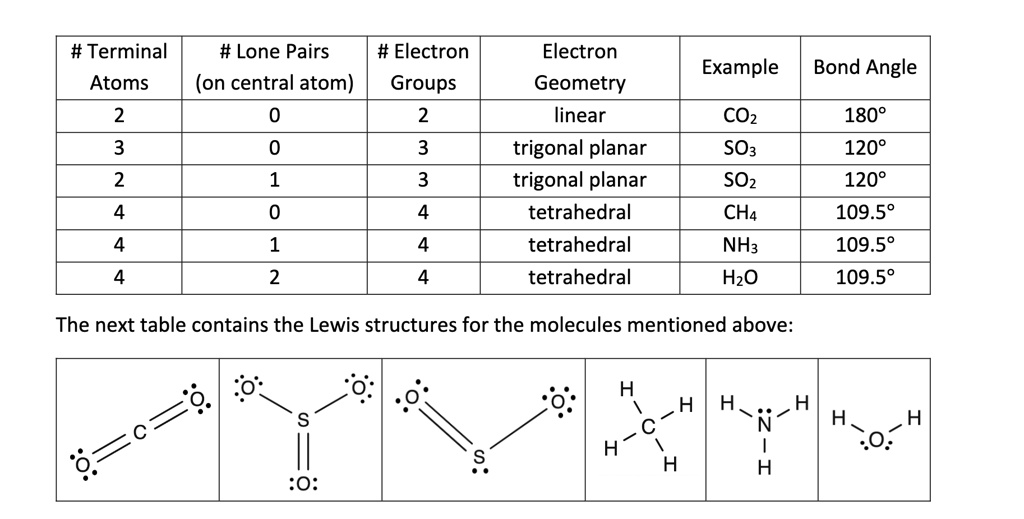
The bent molecular shape is a type of VSEPR geometry (Valence Shell Electron Pair Repulsion) where the central atom forms bonds with two other atoms and has one lone pair of electrons. This arrangement results in a bond angle of approximately 120 degrees, though it can vary depending on the electronegativity of the atoms involved. Common examples include water (H₂O) and sulfur dioxide (SO₂), which exhibit bent shapes due to their electron configurations.
Key Factors Influencing Bent Shape
- Lone Pair Repulsion: Lone pairs occupy more space than bonding pairs, pushing the bonded atoms closer together.
- Electronegativity: Differences in electronegativity between atoms can distort the bond angle.
- Molecular Polarity: Bent molecules are often polar due to the uneven distribution of charge.
📌 Note: Understanding these factors is essential for predicting molecular behavior in reactions and physical properties.
Applications of Bent Molecular Geometry

The bent shape is not just a theoretical concept; it has practical applications in various scientific and industrial fields. From pharmaceuticals to environmental science, molecules with bent geometry play critical roles.
Industries Utilizing Bent Molecules
| Industry | Application |
|---|---|
| Pharmaceuticals | Designing drugs with specific molecular shapes for targeted therapy. |
| Environmental Science | Studying pollutants like SO₂ and their impact on the atmosphere. |
| Material Science | Developing materials with unique properties based on molecular geometry. |
For those interested in commercial applications, understanding bent chemistry shapes can open doors to innovative product development and research opportunities.
How to Identify Bent Molecular Geometry

Identifying a bent shape involves analyzing the Lewis structure and applying VSEPR theory. Here’s a step-by-step guide:
- Draw the Lewis Structure: Determine the arrangement of atoms and lone pairs.
- Apply VSEPR Theory: Use the theory to predict the molecular shape based on electron pair repulsion.
- Calculate Bond Angle: Measure the angle between the bonded atoms, typically around 120 degrees.
📌 Note: Practice with examples like H₂O and SO₂ to master this skill.
Summary and Checklist

To recap, the bent chemistry shape is a fundamental concept in molecular geometry with wide-ranging applications. Here’s a quick checklist to ensure you’ve mastered the topic:
- Understand the role of lone pairs and bonding pairs in forming bent shapes.
- Identify bent molecules using Lewis structures and VSEPR theory.
- Explore applications in industries like pharmaceuticals and environmental science.
In summary, the bent chemistry shape is a cornerstone of molecular geometry, influencing everything from chemical reactions to industrial applications. By understanding its principles, you can predict molecular behavior and explore innovative solutions in various fields. Whether you're studying chemistry basics or advancing in research, mastering this concept will undoubtedly enhance your knowledge and skills.
What causes a molecule to have a bent shape?
+
A molecule adopts a bent shape due to the presence of a lone pair on the central atom, which repels the bonding pairs, causing the atoms to bend.
How does electronegativity affect bent molecules?
+
Higher electronegativity differences between atoms can distort the bond angle, making it slightly less than the ideal 120 degrees.
Can bent molecules be nonpolar?
+
Bent molecules are typically polar due to their asymmetrical shape, but if the outer atoms are the same and the central atom is nonpolar, it can be nonpolar.



