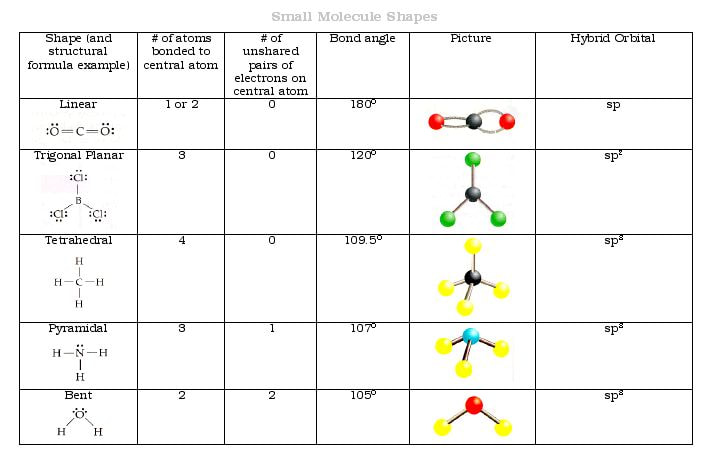
Understanding Bent Geometry Molecules: Structure & Properties

<!DOCTYPE html>
Bent geometry molecules are a fascinating class of chemical compounds characterized by their distinctive V-shaped structure. This unique arrangement arises from the presence of a central atom bonded to two other atoms or groups, with a lone pair of electrons occupying the remaining space. Understanding the structure and properties of bent molecules is crucial in fields like chemistry, materials science, and biology, as they play significant roles in various chemical reactions and biological processes. (Molecular Geometry, Chemical Bonding, VSEPR Theory)
What is Bent Molecular Geometry?

Bent molecular geometry, also known as angular or V-shaped geometry, is a type of molecular structure where the bond angles between the atoms are less than 180 degrees. This geometry is typically observed in molecules with a central atom that has two bonding pairs and one lone pair of electrons. The lone pair repels the bonding pairs, causing the molecule to adopt a bent shape. (VSEPR Theory, Electron Pair Repulsion, Bond Angles)
Key Characteristics of Bent Molecules

Bond Angles
In bent molecules, the bond angles are typically around 104.5 degrees, as seen in water (H₂O). This angle is a result of the lone pair electrons pushing the bonding pairs closer together. (Water Molecule, Bond Angle, Lone Pair)
Polarity
Bent molecules are often polar due to the uneven distribution of charge. The presence of a lone pair on the central atom creates a partial negative charge, while the bonded atoms carry partial positive charges. (Molecular Polarity, Dipole Moment, Chemical Polarity)
Examples of Bent Molecules
- Water (H₂O): A classic example with two O-H bonds and two lone pairs on the oxygen atom.
- Sulfur Dioxide (SO₂): Features one S-O double bond and one S-O single bond, with a lone pair on sulfur.
- Hydrogen Sulfide (H₂S): Similar to water but with sulfur as the central atom.
Properties of Bent Geometry Molecules
Physical Properties
Bent molecules often exhibit high boiling and melting points due to their polarity, which allows for strong intermolecular forces like hydrogen bonding. (Intermolecular Forces, Hydrogen Bonding, Physical Properties)
Chemical Reactivity
The lone pair on the central atom makes bent molecules reactive in various chemical processes, such as nucleophilic substitution and acid-base reactions. (Chemical Reactivity, Nucleophilic Substitution, Acid-Base Reactions)
| Molecule | Bond Angle | Polarity |
|---|---|---|
| H₂O | 104.5° | Polar |
| SO₂ | 119° | Polar |
| H₂S | 92° | Polar |

📌 Note: The bond angles in bent molecules can vary slightly depending on the electronegativity of the atoms involved.
Applications of Bent Molecules

Bent molecules are essential in various applications, including: - Biological Systems: Water’s bent structure is vital for its role as a solvent in living organisms. (Biological Solvent, Water in Biology) - Environmental Chemistry: Sulfur dioxide plays a role in atmospheric chemistry and pollution control. (Atmospheric Chemistry, Pollution Control) - Industrial Processes: Hydrogen sulfide is used in the production of sulfuric acid and heavy water. (Industrial Chemistry, Sulfuric Acid Production)
Checklist for Identifying Bent Molecules
- Check for a central atom with two bonding pairs and one lone pair.
- Measure bond angles to confirm they are less than 180 degrees.
- Assess polarity by examining the electronegativity differences between atoms.
Bent geometry molecules, with their unique structure and properties, are fundamental to understanding chemical behavior. From water’s role in life to sulfur dioxide’s impact on the environment, these molecules are indispensable in both natural and industrial contexts. (Molecular Geometry, Chemical Properties, Industrial Applications)
What causes the bent shape in molecules?
+The bent shape is caused by the repulsion between the lone pair of electrons and the bonding pairs on the central atom, as predicted by VSEPR theory. (VSEPR Theory, Electron Pair Repulsion)
Are all bent molecules polar?
+Yes, most bent molecules are polar due to the uneven distribution of charge caused by the lone pair and the difference in electronegativity between atoms. (Molecular Polarity, Electronegativity)
How do bent molecules differ from linear molecules?
+Bent molecules have bond angles less than 180 degrees due to a lone pair on the central atom, while linear molecules have bond angles of 180 degrees with no lone pairs. (Molecular Geometry, Bond Angles)



