Understanding Binary Stable Compounds: A Comprehensive Guide

Binary stable compounds are fundamental in chemistry, forming the backbone of many scientific and industrial applications. These compounds, composed of two elements in a fixed ratio, exhibit unique properties that make them essential in fields ranging from electronics to medicine. Understanding their structure, formation, and applications is crucial for students, researchers, and professionals alike. Whether you're exploring binary compounds in chemistry for academic purposes or seeking commercial applications of binary compounds, this guide provides a comprehensive overview tailored to your needs.
What Are Binary Stable Compounds?

Binary stable compounds are chemical substances formed by the combination of two elements in a definite proportion. Unlike unstable compounds, they maintain their composition and properties under normal conditions. Examples include sodium chloride (NaCl) and water (H₂O). These compounds are classified based on their bonding types, such as ionic, covalent, or metallic, which determine their physical and chemical characteristics.
💡 Note: Binary compounds are distinct from mixtures, as they have a fixed chemical formula and homogeneous properties.
How Are Binary Stable Compounds Formed?

The formation of binary stable compounds involves chemical reactions where atoms of two elements share or transfer electrons to achieve stability. Key processes include:
- Direct Combination: Elements react directly to form a compound (e.g., hydrogen and oxygen forming water).
- Decomposition: Breaking down a compound into simpler substances.
- Displacement: One element replaces another in a compound.
Understanding these mechanisms is vital for binary compound synthesis in both laboratory and industrial settings.
Types of Binary Stable Compounds
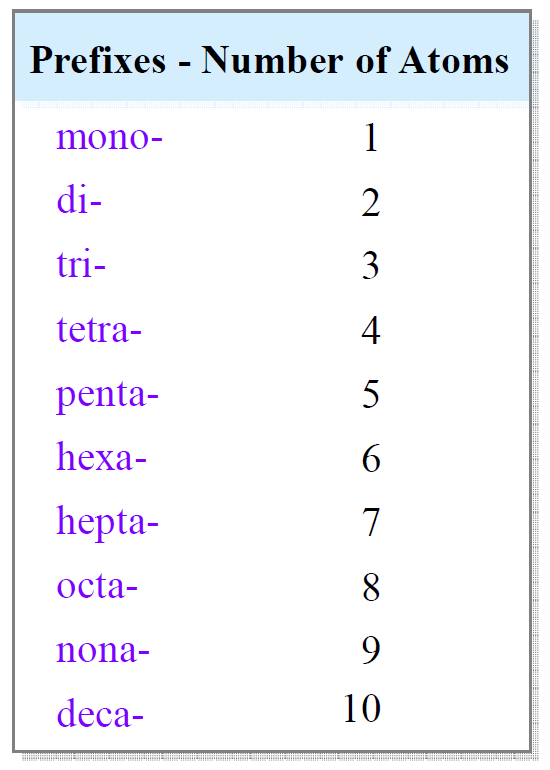
Binary compounds are categorized based on their bonding and composition. Common types include:
| Type | Example | Properties |
|---|---|---|
| Ionic | NaCl (Sodium Chloride) | High melting point, conducts electricity in solution |
| Covalent | H₂O (Water) | Low melting point, poor electrical conductivity |
| Metallic | Al₂O₃ (Aluminum Oxide) | High strength, used in abrasives |

Each type has unique applications, from ionic compounds in electronics to covalent compounds in pharmaceuticals.
Applications of Binary Stable Compounds
Binary compounds are integral to various industries. Notable applications include:
- Electronics: Silicon dioxide (SiO₂) in semiconductors.
- Medicine: Zinc oxide (ZnO) in sunscreens and antiseptics.
- Construction: Calcium carbonate (CaCO₃) in cement and limestone.
For businesses, sourcing high-quality binary compounds is essential for product efficiency and safety.
Checklist for Working with Binary Compounds

Whether you’re a student or professional, follow this checklist for effective handling:
- Identify the type of binary compound (ionic, covalent, metallic).
- Understand its chemical formula and properties.
- Follow safety protocols for storage and handling.
- Explore binary compound suppliers for commercial needs.
Binary stable compounds are a cornerstone of chemistry, with applications spanning across industries. By understanding their formation, types, and uses, you can leverage their potential in academic research or commercial ventures. Whether you're studying binary compounds in chemistry or seeking industrial applications of binary compounds, this guide equips you with the knowledge to succeed.
What is the difference between binary and ternary compounds?
+
Binary compounds consist of two elements, while ternary compounds contain three elements in a fixed ratio.
How are binary compounds named?
+
Binary compounds are named using the elements’ names, with the more metallic element first, followed by the non-metallic element with an “-ide” suffix (e.g., sodium chloride).
What are the commercial uses of binary compounds?
+
Binary compounds are used in electronics (SiO₂), medicine (ZnO), construction (CaCO₃), and more, making them essential for various industries.


