Boltzmann Constant in eV: Quick Conversion Guide

<!DOCTYPE html>
Understanding the Boltzmann constant in eV is essential for anyone working in physics, chemistry, or engineering. This fundamental constant bridges the gap between temperature and energy, making it a cornerstone in thermodynamics and statistical mechanics. Whether you’re a student, researcher, or professional, knowing how to convert the Boltzmann constant to electron volts (eV) can simplify complex calculations. This guide provides a clear, step-by-step explanation to help you master this conversion effortlessly. (Boltzmann constant, eV conversion, thermodynamics)
What is the Boltzmann Constant?
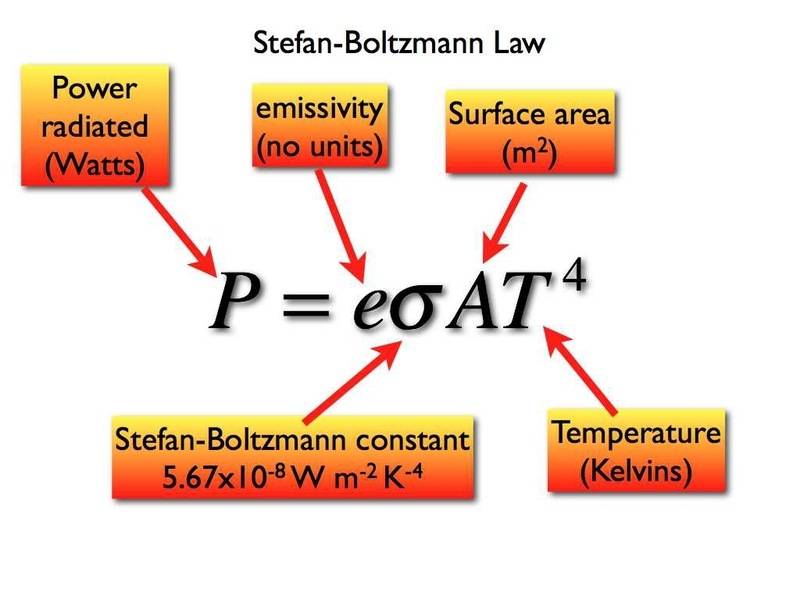
The Boltzmann constant (kB) is a fundamental physical constant that relates the average kinetic energy of particles in a gas to the temperature of the gas. Its value in SI units is approximately 1.38 × 10-23 J/K. However, in many scientific and engineering applications, it’s more convenient to express this constant in electron volts (eV). This conversion is particularly useful in semiconductor physics, quantum mechanics, and material science. (Boltzmann constant definition, SI units, electron volts)
Converting Boltzmann Constant to eV: Step-by-Step Guide
To convert the Boltzmann constant from joules per kelvin (J/K) to electron volts per kelvin (eV/K), follow these steps:
- Step 1: Understand the Conversion Factor – 1 eV is equivalent to 1.602 × 10-19 joules.
- Step 2: Apply the Conversion – Divide the Boltzmann constant in J/K by the conversion factor to get the value in eV/K.
- Step 3: Calculate the Result – The Boltzmann constant in eV/K is approximately 8.617 × 10-5 eV/K.
📌 Note: Always double-check your units to ensure accuracy in scientific calculations. (eV to joules conversion, Boltzmann constant in eV/K)
Why Use Boltzmann Constant in eV?

Expressing the Boltzmann constant in eV offers several advantages:
- Simplified Calculations – eV is a common unit in atomic and molecular physics, making equations easier to handle.
- Relevance in Semiconductors – In semiconductor devices, energy levels are often measured in eV, aligning with the Boltzmann constant in this unit.
- Consistency in Quantum Mechanics – Many quantum mechanical phenomena are described in eV, making this conversion essential for theoretical and experimental work. (semiconductor physics, quantum mechanics, energy levels)
Handy Conversion Table

| Unit | Value |
|---|---|
| Boltzmann Constant (J/K) | 1.38 × 10-23 |
| Boltzmann Constant (eV/K) | 8.617 × 10-5 |
| 1 eV in Joules | 1.602 × 10-19 |
This table provides a quick reference for common conversions related to the Boltzmann constant. (conversion table, units, quick reference)
Practical Applications of Boltzmann Constant in eV

The Boltzmann constant in eV is widely used in:
- Thermodynamics – Relating temperature to energy in statistical mechanics.
- Material Science – Studying thermal properties of materials at the atomic level.
- Electronics – Designing and analyzing semiconductor devices. (thermodynamics, material science, electronics)
Mastering the conversion of the Boltzmann constant to eV is a valuable skill for anyone working in scientific and engineering fields. By following this guide, you’ll be able to seamlessly integrate this constant into your calculations, ensuring accuracy and efficiency in your work. Whether you’re dealing with thermodynamics, quantum mechanics, or semiconductor physics, this knowledge will prove indispensable. (Boltzmann constant in eV, scientific calculations, engineering fields)
What is the Boltzmann constant used for?
+The Boltzmann constant is used to relate temperature to the kinetic energy of particles in a gas and is essential in thermodynamics and statistical mechanics. (Boltzmann constant applications)
How do you convert J/K to eV/K?
+Divide the value in J/K by the conversion factor 1.602 × 10-19 J/eV to get the value in eV/K. (J/K to eV/K conversion)
Why is the Boltzmann constant important in semiconductors?
+It helps relate temperature to energy levels in semiconductor materials, which is crucial for device design and analysis. (semiconductor physics, energy levels)



