Methanol Chemical Structure: A Simple, Clear Breakdown
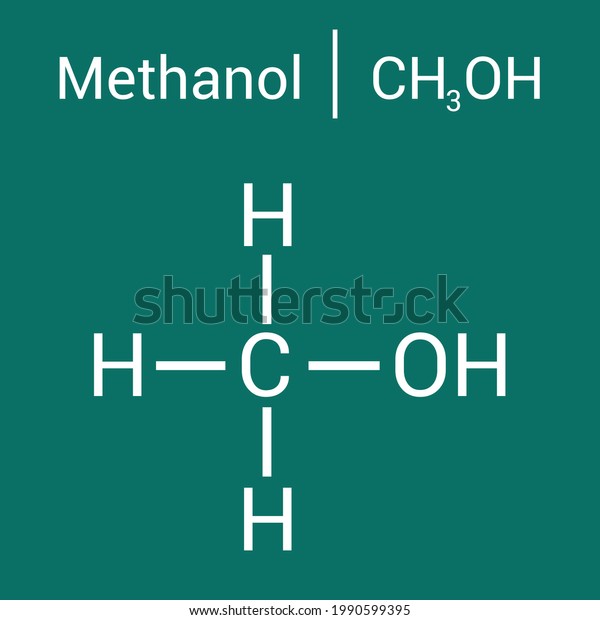
Methanol, also known as wood alcohol, is a simple yet versatile chemical compound with the formula CH₃OH. It plays a crucial role in various industries, from fuel production to chemical manufacturing. Understanding its chemical structure is essential for anyone interested in its applications or properties. Let’s break it down in a clear, concise manner.
What is Methanol’s Chemical Structure?
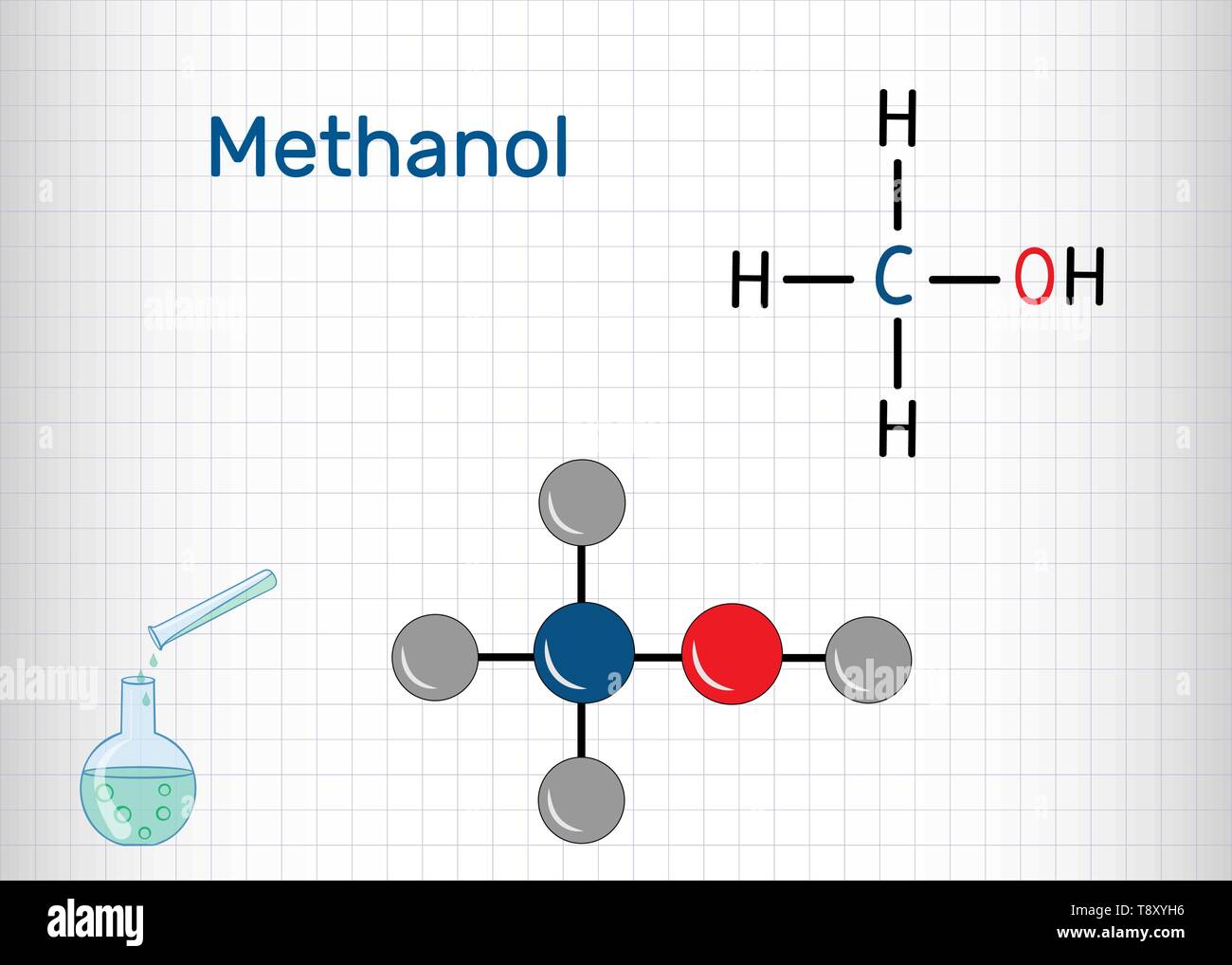
Methanol consists of one carbon atom (C), one oxygen atom (O), and four hydrogen atoms (H). The structure can be visualized as a methane molecule (CH₄) where one hydrogen atom is replaced by a hydroxyl group (-OH). This simple arrangement gives methanol its unique properties, such as being a polar molecule and a protic solvent.
| Atom | Number |
|---|---|
| Carbon (C) | 1 |
| Oxygen (O) | 1 |
| Hydrogen (H) | 4 |
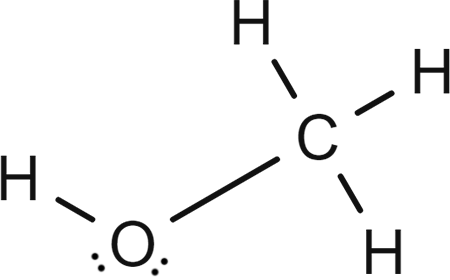
Key Properties of Methanol’s Structure
Methanol’s structure explains its key characteristics:
- Polarity: The -OH group makes it polar, allowing it to dissolve in water and other polar solvents.
- Acidity: It can donate a proton (H⁺), making it a weak acid.
- Reactivity: The structure enables methanol to participate in various chemical reactions, such as esterification and oxidation.
📌 Note: Methanol’s polarity and reactivity make it a valuable solvent and reagent in industrial processes, methanol production, and chemical synthesis.
Applications of Methanol’s Structure

Methanol’s simple structure supports its wide range of applications:
- Fuel: Used as a biofuel or fuel additive.
- Chemical Feedstock: A key ingredient in producing formaldehyde, acetic acid, and other chemicals.
- Solvent: Widely used in laboratories and industries for dissolving substances.
Safety Considerations

While methanol’s structure makes it useful, it’s also toxic if ingested or inhaled. Proper handling and storage are essential to avoid health risks.
⚠️ Note: Always follow safety guidelines when working with methanol, including wearing protective gear and ensuring proper ventilation.
Methanol’s chemical structure, CH₃OH, is simple yet powerful, enabling its use in diverse fields. Its polarity, reactivity, and versatility make it a cornerstone in chemistry and industry. Whether you’re exploring methanol production or its applications, understanding its structure is the first step to harnessing its potential.
What is the chemical formula of methanol?
+The chemical formula of methanol is CH₃OH.
Why is methanol considered a polar molecule?
+Methanol is polar due to the presence of the -OH group, which creates an uneven distribution of charge.
What are the main uses of methanol?
+Methanol is used as a fuel, chemical feedstock, and solvent in various industries.
Related Keywords: methanol production, chemical synthesis, industrial solvents, methanol properties, methanol safety.



