Understanding CO₂ Intermolecular Forces: A Quick Guide

<!DOCTYPE html>
Carbon dioxide (CO₂) is a vital molecule with unique properties, largely due to its intermolecular forces. Understanding these forces is crucial for grasping CO₂’s behavior in various states and its role in climate science, industrial applications, and more. This guide breaks down the key concepts in a clear, SEO-friendly manner, catering to both informational and commercial audiences.
What Are Intermolecular Forces in CO₂?
Intermolecular forces are the attractions between molecules. In CO₂, these forces are primarily London dispersion forces (a type of van der Waals force) and dipole-induced dipole interactions. Unlike hydrogen bonding or permanent dipole-dipole forces, CO₂’s linear, symmetrical structure results in a nonpolar molecule, limiting its intermolecular forces to weaker types.
💡 Note: CO₂’s lack of polarity means it relies on dispersion forces, which increase with molecular size and complexity.
Types of Intermolecular Forces in CO₂

1. London Dispersion Forces
These forces arise from temporary fluctuations in electron distribution, creating instantaneous dipoles. In CO₂, these are the dominant intermolecular forces due to its nonpolar nature.
2. Dipole-Induced Dipole Interactions
While CO₂ is nonpolar, it can be temporarily distorted by polar molecules nearby, leading to weak dipole-induced dipole interactions.
How Intermolecular Forces Affect CO₂’s Properties

The weak intermolecular forces in CO₂ explain its low boiling point (-78.5°C) and its existence as a gas at room temperature. These forces also influence its solubility in water and its role in the Earth’s atmosphere.
| Property | Explanation |
|---|---|
| Low Boiling Point | Weak forces require less energy to break, allowing CO₂ to vaporize easily. |
| Solubility in Water | CO₂ dissolves in water due to dipole-induced dipole interactions, forming carbonic acid. |

Applications of CO₂ Intermolecular Forces

Understanding these forces is essential for industries like carbon capture technology, food preservation, and chemical manufacturing. For commercial audiences, this knowledge aids in optimizing processes involving CO₂, such as supercritical fluid extraction or enhanced oil recovery.
Checklist for Understanding CO₂ Intermolecular Forces
- Identify the primary intermolecular forces in CO₂ (London dispersion forces).
- Understand how these forces influence CO₂’s physical properties.
- Apply this knowledge to real-world applications like carbon capture or industrial processes.
By mastering CO₂’s intermolecular forces, you’ll gain insights into its behavior and applications, whether for academic research or industrial innovation. (CO₂ properties, intermolecular forces, carbon capture technology)
Why is CO₂ a gas at room temperature?
+CO₂ is a gas at room temperature due to its weak intermolecular forces (London dispersion forces), which require minimal energy to overcome.
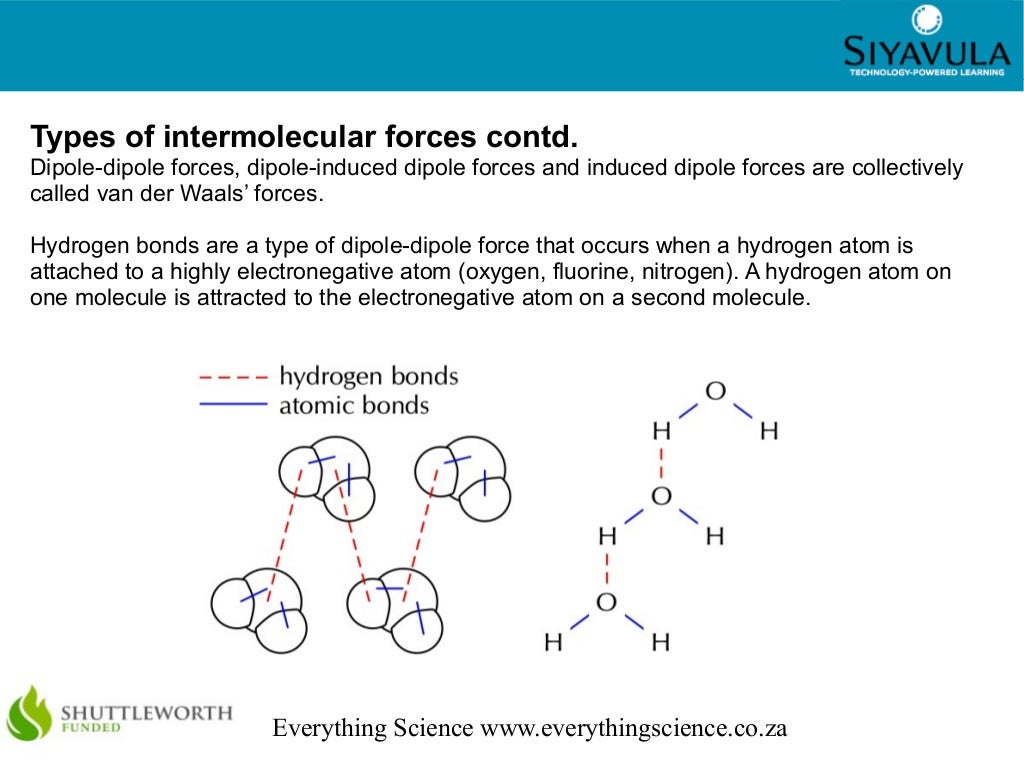
Can CO₂ form hydrogen bonds?
+No, CO₂ cannot form hydrogen bonds because it lacks a hydrogen atom bonded to a highly electronegative atom like oxygen or nitrogen.
How do intermolecular forces affect CO₂ solubility?
+CO₂ dissolves in water through dipole-induced dipole interactions, forming carbonic acid, despite its nonpolar nature.


