Does SCN Have Resonance? Uncover the Truth!
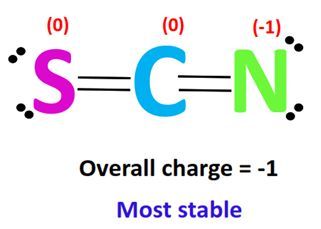
When it comes to chemical compounds, the concept of resonance often sparks curiosity. One such compound that frequently arises in discussions is SCN (thiocyanate). But does SCN have resonance? Understanding this not only satisfies scientific curiosity but also has practical implications in fields like chemistry and biology. In this post, we’ll delve into the structure of SCN, explore the concept of resonance, and uncover whether SCN exhibits this phenomenon. (Resonance in chemistry, SCN structure, thiocyanate properties)
What is Resonance in Chemistry?
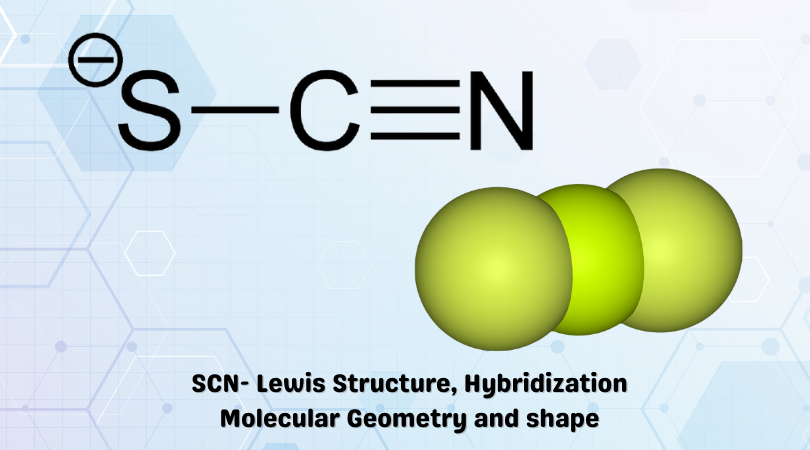
Resonance is a key concept in chemistry that describes the delocalization of electrons in a molecule. It occurs when a single structure cannot fully represent a molecule’s bonding, and multiple structures (resonance structures) are used to depict its electron distribution. Resonance stabilizes molecules and influences their reactivity. (Chemical resonance, electron delocalization, molecular stability)
Key Characteristics of Resonance
- Delocalized Electrons: Electrons are not confined to a single bond or atom.
- Multiple Structures: Resonance hybrids are used to represent the molecule.
- Stability: Resonance often leads to lower energy states and increased stability.
The Structure of SCN (Thiocyanate)

SCN, or thiocyanate, consists of sulfur, carbon, and nitrogen atoms. Its structure is represented as S-C≡N, with a triple bond between carbon and nitrogen and a single bond between sulfur and carbon. This linear arrangement raises questions about its potential for resonance. (SCN molecular structure, thiocyanate bonding, linear molecules)
Bonding in SCN
| Atom | Bond Type |
|---|---|
| Sulfur (S) | Single Bond to Carbon |
| Carbon © | Triple Bond to Nitrogen |
| Nitrogen (N) | Part of Triple Bond |

📌 Note: The linear structure of SCN is crucial in determining its resonance behavior.
Does SCN Exhibit Resonance?
To determine if SCN has resonance, we examine its electron distribution. While the triple bond between carbon and nitrogen is stable, the single bond between sulfur and carbon limits significant electron delocalization. Therefore, SCN does not exhibit classic resonance like molecules with delocalized π electrons (e.g., benzene). (SCN resonance, electron delocalization in SCN, thiocyanate stability)
Why SCN Lacks Resonance
- Linear Geometry: Limits electron movement.
- Localized Bonds: Electrons are confined to specific bonds.
- No π Electron Cloud: Unlike aromatic compounds, SCN lacks a delocalized π system.
Checklist: Understanding SCN and Resonance
- Review the structure of SCN (S-C≡N).
- Understand the concept of resonance in chemistry.
- Identify why SCN does not exhibit resonance.
- Compare SCN with molecules that show resonance (e.g., benzene).
In summary, while SCN is a fascinating molecule with unique bonding, it does not exhibit resonance due to its linear structure and localized electrons. Understanding this helps clarify its behavior in chemical reactions and its role in various applications. Whether you’re a student or a professional, grasping these concepts enhances your knowledge of molecular chemistry. (SCN resonance, thiocyanate properties, molecular chemistry)
What is resonance in chemistry?
+
Resonance is the delocalization of electrons in a molecule, represented by multiple structures called resonance hybrids, which stabilize the molecule.
Why doesn’t SCN exhibit resonance?
+
SCN’s linear structure and localized bonds prevent significant electron delocalization, a key requirement for resonance.
What are examples of molecules with resonance?
+
Benzene, ozone (O₃), and nitrate ion (NO₃⁻) are classic examples of molecules exhibiting resonance.