CH2O Electron Geometry: Shape & Structure Explained Understanding the Electron Geometry of CH2O What is the Electron Geometry of CH2O? CH2O: Unraveling Its Electron Geometry Exploring the Electron Geometry of Formaldehyde (CH2O)

Formaldehyde, or CH2O, is a simple yet fascinating molecule with significant implications in chemistry and biology. Understanding its electron geometry is crucial for grasping its reactivity and applications. In this post, we’ll explore the CH2O electron geometry, its shape, and molecular structure, breaking down complex concepts into digestible insights.
Understanding the Electron Geometry of CH2O
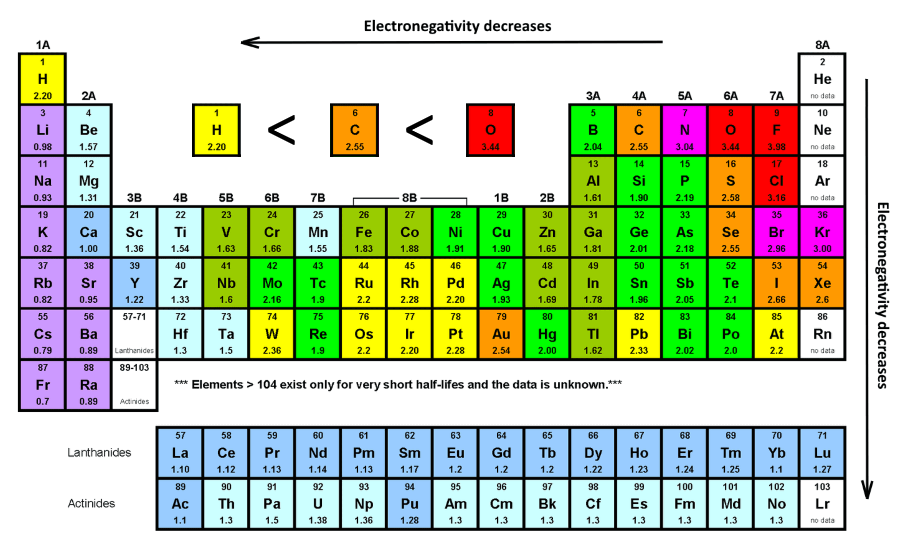
The electron geometry of CH2O is determined by its central carbon atom, which forms bonds with two hydrogen atoms and one oxygen atom. To understand its geometry, we apply the VSEPR theory (Valence Shell Electron Pair Repulsion), which predicts the arrangement of electron pairs around the central atom to minimize repulsion.
What is the Electron Geometry of CH2O?
CH2O has a trigonal planar electron geometry. This is because the central carbon atom has three regions of electron density: two single bonds with hydrogen and one double bond with oxygen. The double bond counts as one region of electron density, resulting in a trigonal planar arrangement.
CH2O: Unraveling Its Electron Geometry
Let’s break it down further. The molecular geometry of CH2O is trigonal planar, with bond angles of approximately 120 degrees. The double bond between carbon and oxygen makes the molecule polar, as oxygen is more electronegative than carbon, creating a partial negative charge on the oxygen atom and a partial positive charge on the carbon atom.
| Aspect | Description |
|---|---|
| Electron Geometry | Trigonal Planar |
| Molecular Geometry | Trigonal Planar |
| Bond Angles | ~120 degrees |
| Polarity | Polar (due to C=O bond) |

Exploring the Electron Geometry of Formaldehyde (CH2O)

Formaldehyde’s trigonal planar geometry has practical implications. Its polar nature makes it soluble in water and reactive with other polar molecules. This property is leveraged in industries like preservation, disinfection, and chemical synthesis. Understanding its geometry helps chemists predict its behavior in reactions.
💡 Note: The double bond in CH2O contributes to its reactivity, making it a versatile molecule in organic chemistry.
In summary, CH2O’s electron geometry is trigonal planar, with a central carbon atom bonded to two hydrogen atoms and one oxygen atom via a double bond. Its polar nature and 120-degree bond angles make it a key player in various chemical processes. Whether you’re a student or a professional, mastering CH2O’s geometry is essential for understanding its applications and reactivity.
What is the electron geometry of CH2O?
+The electron geometry of CH2O is trigonal planar, determined by its three regions of electron density around the central carbon atom.
Why is CH2O polar?
+CH2O is polar due to the electronegativity difference between carbon and oxygen in the C=O double bond, creating partial charges on the molecule.
What are the bond angles in CH2O?
+The bond angles in CH2O are approximately 120 degrees, characteristic of its trigonal planar geometry.
Related: VSEPR Theory Explained, Polar vs Nonpolar Molecules, Applications of Formaldehyde