Fluorine Bohr Model: A Simple Visualization Explained Understanding Fluorine’s Bohr Model: Structure & Key Facts Bohr Model of Fluorine: Atomic Insights Made Easy Fluorine’s Bohr Model: Atomic Structure Simplified Exploring the Bohr Model of Fluorine Atom

Understanding the Fluorine Bohr Model is essential for anyone studying atomic structure or chemistry. This simple yet powerful visualization helps us grasp how fluorine’s electrons are arranged around its nucleus. Whether you’re a student, educator, or simply curious about atomic models, this guide breaks down the Bohr Model of Fluorine into easy-to-understand insights. From its atomic structure to key facts, we’ll explore everything you need to know to master this topic, Fluorine Bohr Model, Bohr Model of Fluorine, Fluorine’s Atomic Structure.
Understanding Fluorine’s Bohr Model: Structure & Key Facts

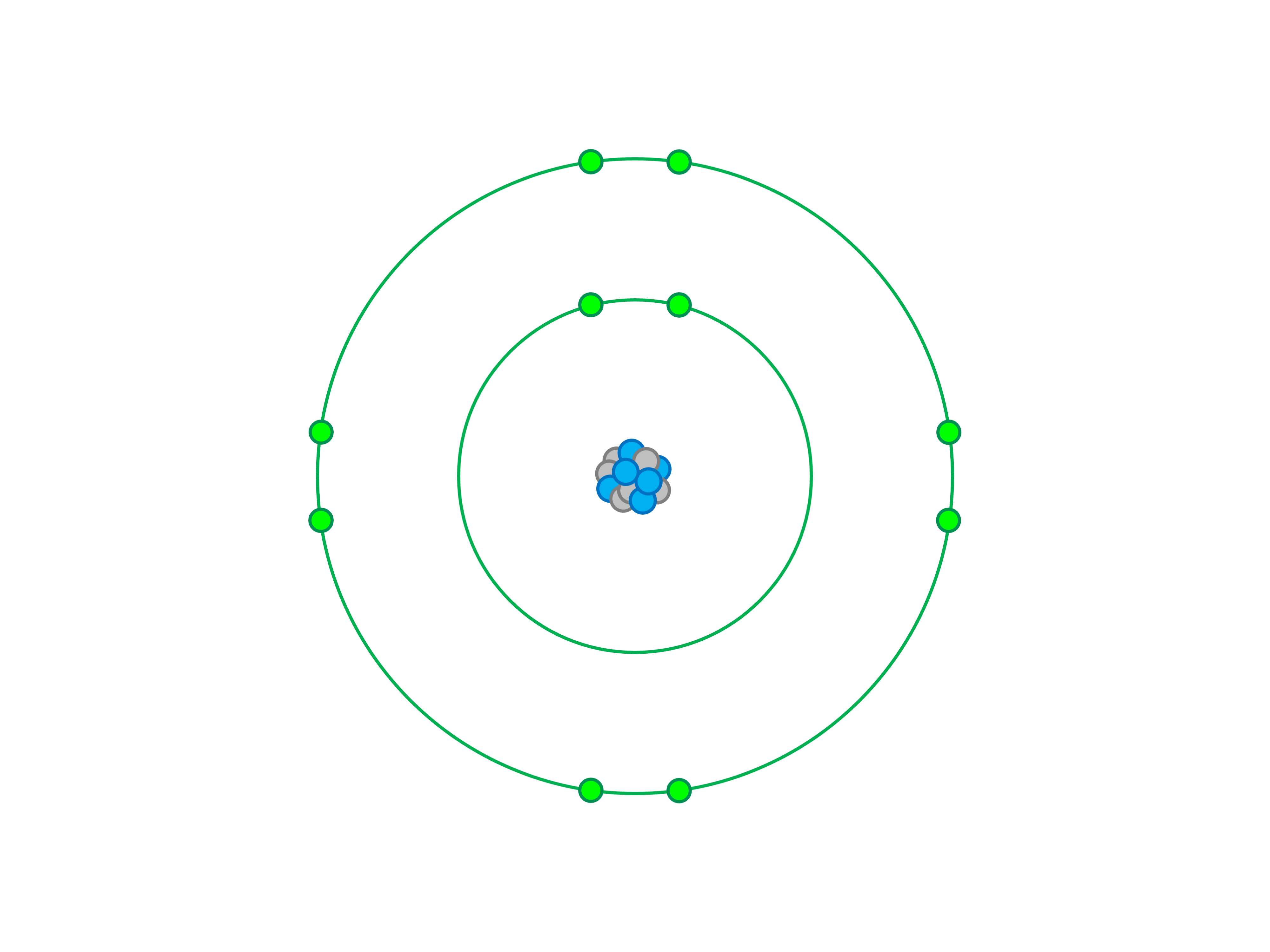
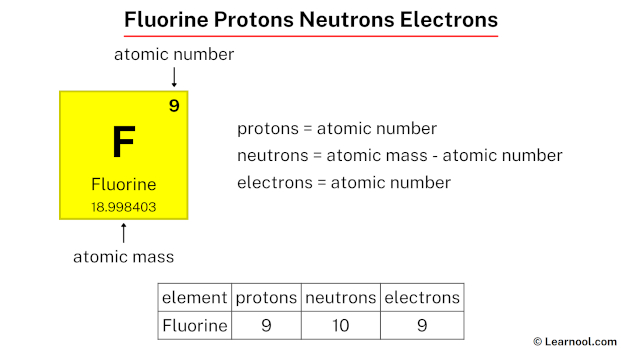
The Bohr Model of Fluorine illustrates the arrangement of its 9 electrons in energy levels or shells. Fluorine, with an atomic number of 9, has a nucleus containing 9 protons and 9 neutrons, surrounded by electrons. The first shell holds 2 electrons, while the second shell contains the remaining 7 electrons. This configuration, written as 2-7, is crucial for understanding fluorine’s chemical behavior, Fluorine Bohr Model, Fluorine’s Atomic Structure.
Why the Bohr Model Matters
The Bohr Model simplifies complex atomic structures, making it easier to visualize electron distribution. For fluorine, this model explains its high reactivity due to its outermost electron configuration. By understanding this model, you can predict how fluorine interacts with other elements, Fluorine Bohr Model, Bohr Model of Fluorine.
Bohr Model of Fluorine: Atomic Insights Made Easy
Let’s dive deeper into the Fluorine Bohr Model. Fluorine’s electron configuration is 1s² 2s² 2p⁵, meaning it has 2 electrons in the first shell and 7 in the second. The 2p⁵ configuration highlights its need for one more electron to achieve a stable octet, making it highly reactive, Fluorine’s Atomic Structure, Fluorine Bohr Model.
| Shell | Electrons |
|---|---|
| First (K) | 2 |
| Second (L) | 7 |

Visualizing the Model
Imagine the Bohr Model of Fluorine as a series of concentric circles (shells) around the nucleus. The inner circle represents the first shell with 2 electrons, while the outer circle holds the remaining 7 electrons. This visual representation simplifies complex atomic theory, Bohr Model of Fluorine, Fluorine Bohr Model.
📌 Note: The Bohr Model is a simplified representation and does not account for advanced quantum mechanics concepts like orbitals.
Fluorine’s Bohr Model: Atomic Structure Simplified
Simplifying the Fluorine Bohr Model helps in grasping its atomic structure. Here’s a quick checklist to remember its key features:
- Atomic Number: 9
- Electron Configuration: 2-7
- Highly Reactive Due to Unstable Outer Shell
This checklist ensures you never miss a detail when studying the Bohr Model of Fluorine, Fluorine Bohr Model, Fluorine’s Atomic Structure.
Exploring the Bohr Model of Fluorine Atom
Exploring the Bohr Model of Fluorine reveals its significance in chemistry. Fluorine’s electron arrangement explains its position in the periodic table and its role in forming compounds. For instance, its reactivity makes it a key component in fluorides and fluorocarbons, Bohr Model of Fluorine, Fluorine Bohr Model.
In summary, the Fluorine Bohr Model provides a clear and simple way to understand fluorine’s atomic structure. By focusing on its electron configuration and reactivity, you can appreciate its role in chemistry. Whether you’re studying for exams or simply expanding your knowledge, mastering this model is a valuable step, Fluorine Bohr Model, Bohr Model of Fluorine, Fluorine’s Atomic Structure.
What is the Bohr Model of Fluorine?
+
The Bohr Model of Fluorine is a simplified visualization of its atomic structure, showing 2 electrons in the first shell and 7 in the second shell.
Why is Fluorine highly reactive?
+
Fluorine is highly reactive because it needs one more electron to complete its outer shell and achieve a stable octet.
How many electrons does Fluorine have?
+
Fluorine has 9 electrons, arranged as 2 in the first shell and 7 in the second shell.



