Understanding Imine Formation Mechanism: A Simple Guide
<!DOCTYPE html>
Imine formation is a fundamental reaction in organic chemistry, widely used in synthesis and biological processes. Understanding its mechanism is crucial for chemists, students, and researchers. This guide breaks down the process into simple steps, making it accessible for both informational and commercial purposes.
What is an Imine? (Imine Definition, Organic Chemistry Basics)

An imine is a functional group with a carbon-nitrogen double bond (C=N), formed by the condensation of an amine and a carbonyl compound. Imine formation is reversible and plays a key role in dynamic systems like drug design and material science.
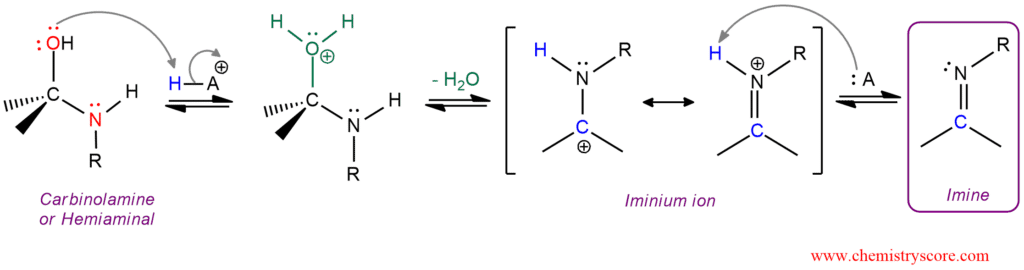
The Mechanism of Imine Formation (Imine Formation Steps, Nucleophilic Addition)

The process begins with a nucleophilic attack by the amine on the carbonyl carbon. This step is followed by proton transfer and water elimination, resulting in the C=N bond. Below is a simplified breakdown:
- Step 1: Nucleophilic attack by the amine on the carbonyl carbon.
- Step 2: Proton transfer to form a hemiaminal intermediate.
- Step 3: Elimination of water to form the imine.
📌 Note: The reaction is acid-catalyzed, enhancing the electrophilicity of the carbonyl carbon.
Factors Affecting Imine Formation (Reaction Conditions, Catalysts)
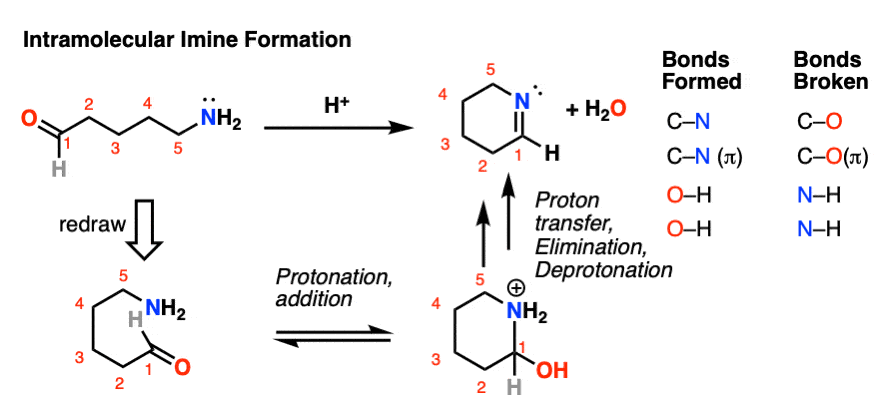
Several factors influence the efficiency of imine formation, including:
| Factor | Impact |
|---|---|
| Temperature | Higher temperatures favor the forward reaction. |
| Catalysts | Acids or molecular sieves accelerate the process. |
| Solvent | Aprotic solvents like dichloromethane are commonly used. |
Applications of Imine Formation (Imine Synthesis, Commercial Uses)

Imine formation is not just a theoretical concept; it has practical applications in:
- Pharmaceuticals: Synthesis of complex drug molecules.
- Materials Science: Development of polymers and dynamic materials.
- Biochemistry: Understanding enzyme mechanisms and biological processes.
For those looking to apply imine formation in commercial settings, optimizing reaction conditions and choosing the right catalysts are essential steps. (Imine Commercial Applications, Organic Synthesis)
Checklist for Successful Imine Formation (Imine Formation Tips, Troubleshooting)

To ensure a successful imine formation, follow these steps:
- Choose the right carbonyl compound and amine.
- Use an appropriate catalyst (e.g., acid or molecular sieves).
- Monitor reaction conditions (temperature, solvent).
- Purify the product using techniques like chromatography.
Mastering imine formation opens doors to advanced organic synthesis and innovative applications in chemistry. Whether you’re a student or a professional, understanding this mechanism is a valuable asset. (Imine Formation Guide, Chemistry Tutorials)
What is the role of acid in imine formation?
+Acid catalyzes the reaction by protonating the carbonyl oxygen, making the carbon more electrophilic and facilitating the nucleophilic attack by the amine.
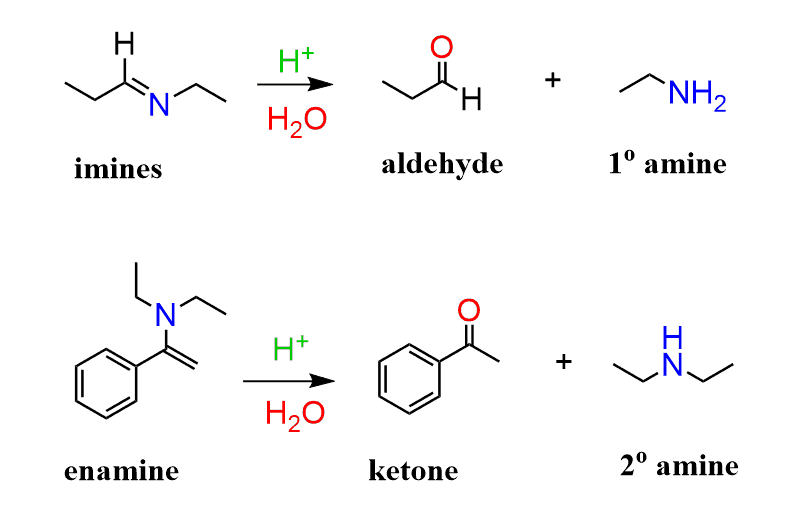
Can imine formation be reversed?
+Yes, imine formation is reversible. Adding water and acid can hydrolyze the imine back to the amine and carbonyl compound.
What solvents are best for imine formation?
+Aprotic solvents like dichloromethane or acetonitrile are commonly used as they do not interfere with the reaction.