IR Bands for Functional Groups: Quick Identification Guide

Opening Paragraph
Infrared (IR) spectroscopy is a powerful tool for identifying functional groups in organic compounds. By analyzing IR bands, chemists can quickly determine the presence of specific functional groups, such as alcohols, carboxylic acids, or alkenes. This guide provides a concise yet comprehensive overview of IR bands for functional groups, helping you master quick identification techniques. Whether you’re a student, researcher, or professional, understanding these bands is essential for accurate compound analysis.
Understanding IR Spectroscopy Basics
IR spectroscopy works by measuring the absorption of infrared light by molecules. Each functional group absorbs light at characteristic wavelengths, known as IR bands. These bands appear as peaks on an IR spectrum, allowing for precise identification. Key regions to focus on include the functional group region (2500–1500 cm⁻¹) and the fingerprint region (1500–400 cm⁻¹).
📌 Note: The functional group region is more diagnostic, while the fingerprint region provides additional structural details.
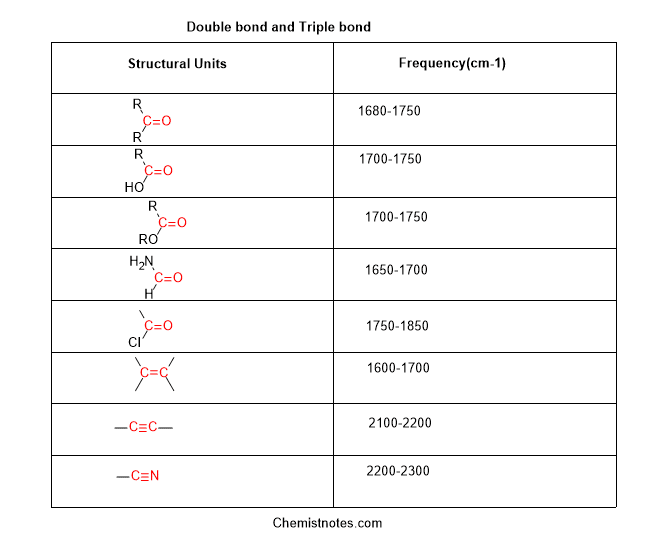
Common IR Bands for Functional Groups

Below is a table summarizing IR bands for functional groups and their characteristic absorptions:
| Functional Group | IR Band (cm⁻¹) | Notes |
|---|---|---|
| Alkane (C-H) | 2960–2850 | Symmetric and asymmetric stretching |
| Alkene (C=C) | 1680–1600 | C=C stretching |
| Alcohol (O-H) | 3600–3200 | Broad peak, hydrogen bonding |
| Carboxylic Acid (O-H) | 3500–2500 | Broad, strong peak |
| Amine (N-H) | 3500–3300 | Sharp or broad peaks |

Quick Identification Tips

To efficiently identify functional groups using IR spectroscopy:
- Focus on strong, sharp peaks in the functional group region.
- Look for broad peaks indicating hydrogen bonding (e.g., alcohols, carboxylic acids).
- Compare spectra with known reference data for accuracy.
✨ Note: Always consider the molecule's context and other spectroscopic data for confirmation.
Checklist for IR Band Identification

Use this checklist to streamline your analysis:
- Identify the region: Functional group or fingerprint?
- Note peak shape: Sharp, broad, or medium?
- Match to known bands: Refer to the table above.
- Consider molecular structure: Does the functional group fit?
Final Thoughts
Mastering IR bands for functional groups is a valuable skill for anyone working in chemistry. By understanding characteristic absorptions and using systematic analysis, you can quickly identify functional groups with confidence. Keep this guide handy and practice regularly to enhance your spectroscopic expertise.
Related Keywords: IR spectroscopy, functional group identification, organic chemistry, spectroscopic analysis
What are IR bands in spectroscopy?
+
IR bands are specific wavelengths at which functional groups absorb infrared light, appearing as peaks on an IR spectrum.
How do I identify an alcohol using IR spectroscopy?
+
Look for a broad O-H stretch peak between 3600–3200 cm⁻¹, indicative of hydrogen bonding in alcohols.
Why is the fingerprint region important?
+
The fingerprint region (1500–400 cm⁻¹) provides unique patterns that help distinguish between similar compounds.



