Is Dissolution a Chemical Change? Quick Facts.
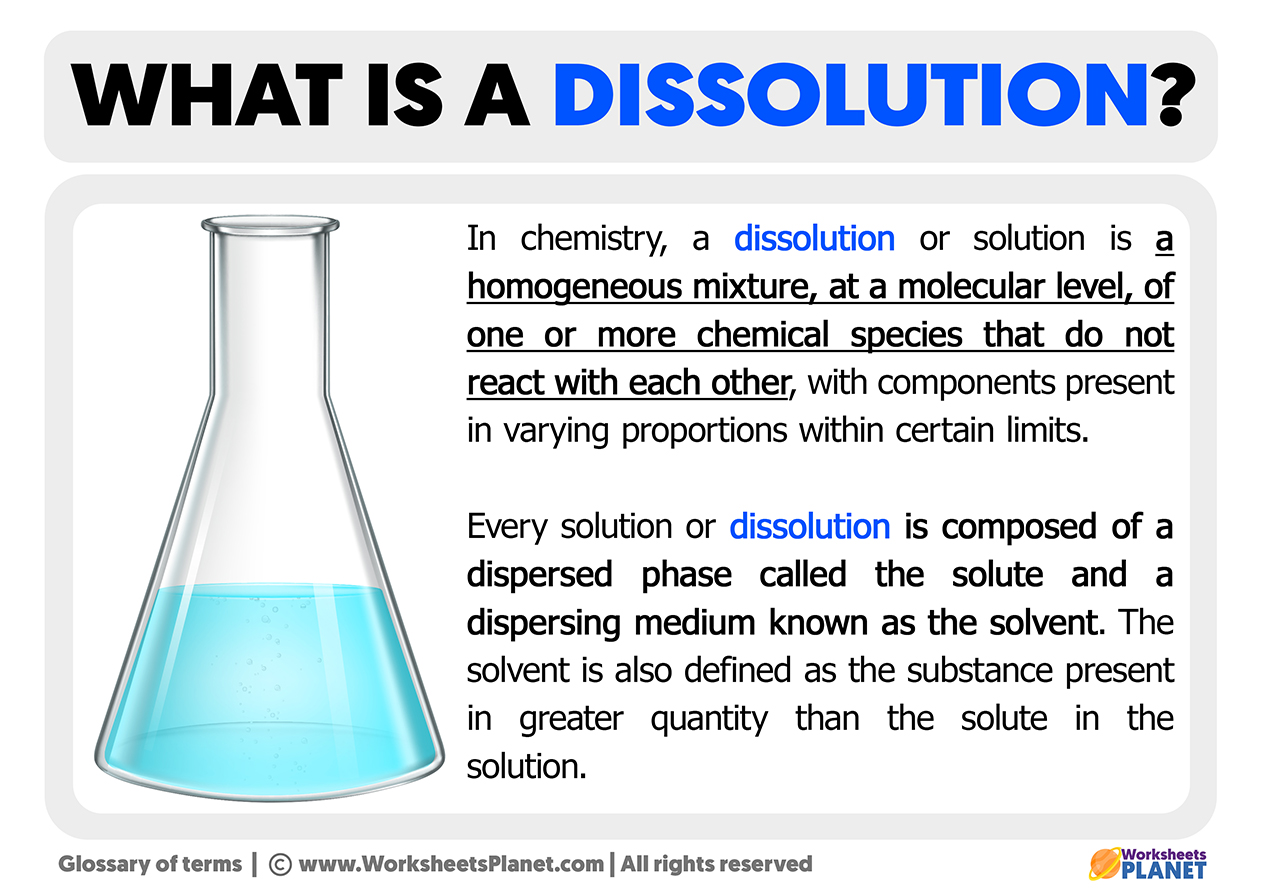
Dissolution is a process that often sparks curiosity in chemistry enthusiasts and students alike. But is dissolution a chemical change? To answer this, we need to understand the nature of dissolution and how it differs from chemical reactions. In simple terms, dissolution involves a substance (the solute) dispersing uniformly into a solvent to form a solution. This process is fundamental in various fields, from pharmaceuticals to environmental science. Let’s dive into the quick facts to clarify whether dissolution qualifies as a chemical change or not.
Understanding Dissolution: What Happens During the Process?

Dissolution occurs when a solute breaks apart at the molecular level and mixes with a solvent. For example, when you stir sugar into water, the sugar molecules disperse evenly, forming a homogeneous mixture. This process is primarily physical, as it does not alter the chemical identity of the solute or solvent. The key takeaway here is that dissolution involves the separation of particles without changing their chemical structure.
Chemical Change vs. Physical Change: Where Does Dissolution Fit?

A chemical change involves the formation of new substances with different properties, while a physical change only alters the form or appearance of a substance. Dissolution is typically a physical change because the solute and solvent retain their chemical identities. However, there are exceptions, such as when a chemical reaction occurs simultaneously during dissolution, like in the case of dissolving metals in acids.
Checklist: Key Indicators of Dissolution
- The solute disperses uniformly in the solvent.
- No new substances are formed.
- The process is usually reversible.
- Energy changes (heat absorption or release) may occur but do not alter chemical identity.
Exceptions to the Rule: When Dissolution Involves Chemical Changes

While dissolution is generally a physical process, certain scenarios involve chemical changes. For instance, dissolving hydrochloric acid in water releases hydrogen and chloride ions, which can participate in chemical reactions. Similarly, dissolving sodium metal in water produces hydrogen gas and sodium hydroxide, clearly indicating a chemical change. These exceptions highlight the importance of context in determining the nature of dissolution.
| Type of Dissolution | Example | Chemical Change? |
|---|---|---|
| Physical Dissolution | Sugar in water | No |
| Chemical Dissolution | Sodium in water | Yes |
📌 Note: Always consider the context of the dissolution process to determine whether it involves a chemical change.
In summary, dissolution is primarily a physical change, as it does not alter the chemical identity of the substances involved. However, exceptions exist, particularly when chemical reactions occur during the process. Understanding this distinction is crucial for applications in chemistry, industry, and everyday life. Whether you’re a student or a professional, grasping the nuances of dissolution will enhance your knowledge of chemical processes.
Is dissolving salt in water a chemical change?
+No, dissolving salt in water is a physical change. The salt (sodium chloride) dissociates into ions but does not form new substances.
Can dissolution ever be reversible?
+Yes, most dissolution processes are reversible. For example, evaporating the solvent can recover the original solute.
What is an example of dissolution with a chemical change?
+Dissolving sodium metal in water produces hydrogen gas and sodium hydroxide, which is a chemical change.
chemical change, physical change, solute, solvent, homogeneous mixture, chemical reactions, dissolution process, sodium metal, hydrochloric acid, chemistry basics.