Is Potassium Hydroxide (KOH) a Weak or Strong Base?
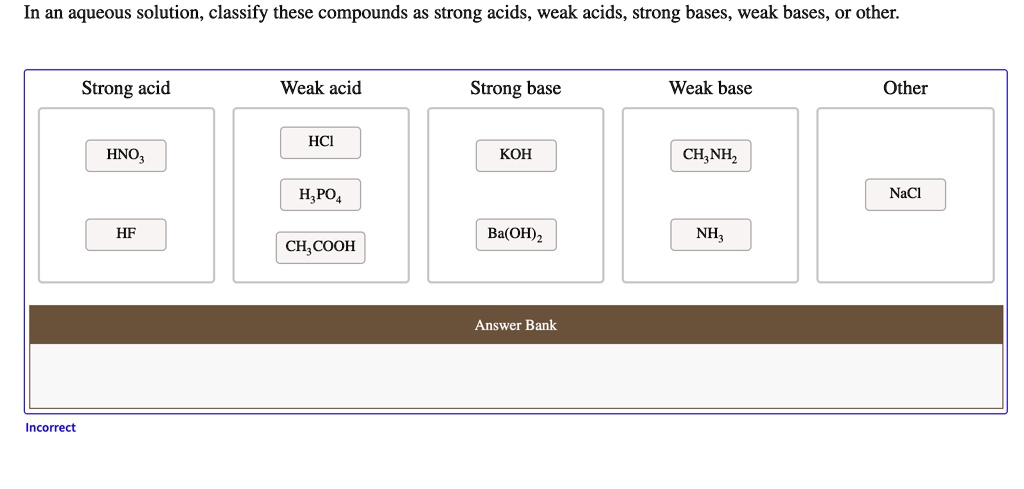
Potassium hydroxide (KOH) is a chemical compound widely used in various industries, from soap-making to biodiesel production. One common question that arises is whether KOH is a weak or strong base. Understanding its classification is crucial for applications in chemistry, manufacturing, and even home DIY projects. In this post, we’ll explore the properties of KOH, its strength as a base, and its practical uses, ensuring you have all the information you need.
What Makes a Base Strong or Weak?
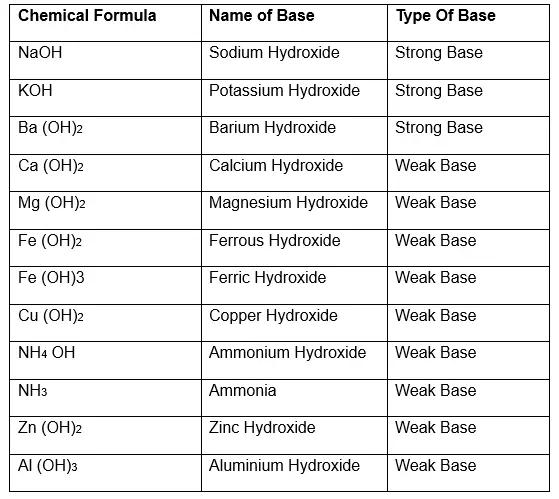
Before diving into KOH, it’s essential to understand the criteria for classifying bases. A strong base fully dissociates in water, releasing a high concentration of hydroxide ions (OH⁻). Examples include sodium hydroxide (NaOH) and, as we’ll see, potassium hydroxide (KOH). On the other hand, a weak base only partially dissociates, releasing fewer hydroxide ions. Examples include ammonia (NH₃).
Is Potassium Hydroxide (KOH) a Strong Base?

Yes, potassium hydroxide (KOH) is a strong base. When dissolved in water, it completely dissociates into potassium ions (K⁺) and hydroxide ions (OH⁻). This full dissociation results in a high concentration of OH⁻ ions, making KOH highly effective in neutralizing acids and performing other base-related functions.
| Property | KOH (Strong Base) | Weak Base (e.g., NH₃) |
|---|---|---|
| Dissociation in Water | Complete | Partial |
| pH Level | High (typically 13-14) | Moderate (typically 8-10) |
| Conductivity | High | Low |

Practical Applications of KOH as a Strong Base

KOH’s strength as a base makes it invaluable in numerous applications:
- Soap Making: KOH is used in the saponification process to convert fats into soap.
- Biodiesel Production: It catalyzes the conversion of vegetable oils into biodiesel.
- Chemical Manufacturing: KOH is used in producing various chemicals, including fertilizers and pharmaceuticals.
- Cleaning Agents: Its strong alkaline nature helps remove grease and stains.
📌 Note: Always handle KOH with care, as it is corrosive and can cause skin burns.
How to Safely Use Potassium Hydroxide

When working with KOH, follow these safety tips:
- Wear protective gloves, goggles, and clothing.
- Work in a well-ventilated area to avoid inhaling fumes.
- Store KOH in a cool, dry place away from acids and flammable materials.
KOH vs. NaOH: Which Strong Base to Choose?

Both KOH and sodium hydroxide (NaOH) are strong bases, but they have distinct uses:
- KOH is preferred in applications requiring a potassium-based product, such as certain soaps and biodiesel.
- NaOH is more commonly used in general cleaning and industrial processes due to its lower cost.
Final Thoughts
Potassium hydroxide (KOH) is undeniably a strong base, making it a powerful tool in various industries. Its ability to fully dissociate in water and release high concentrations of OH⁻ ions distinguishes it from weak bases. Whether you’re a chemist, manufacturer, or DIY enthusiast, understanding KOH’s properties ensures safe and effective use.
Is KOH safe to use at home?
+Yes, but handle with care. Wear protective gear and follow safety guidelines to avoid burns or accidents.
Can KOH be used for cleaning drains?
+Yes, KOH can dissolve grease and clogs, but it’s stronger than typical drain cleaners, so use sparingly.
What’s the difference between KOH and lye?
+Lye is a general term for strong alkaline substances, including KOH and NaOH. KOH is specifically potassium hydroxide.
(strong base classification,potassium hydroxide uses,KOH safety tips,KOH vs NaOH)



