lewis dot diagram for of2

<!DOCTYPE html>
Understanding the Lewis dot diagram for OF2 (oxygen difluoride) is essential for grasping its molecular structure and bonding. This diagram visually represents the arrangement of electrons in the molecule, helping students and chemists predict its reactivity and properties. Whether you’re studying chemistry or need a refresher, this guide breaks down the process step-by-step, ensuring clarity and accuracy.
What is a Lewis Dot Diagram?

A Lewis dot diagram, also known as an electron dot diagram, illustrates the valence electrons of atoms within a molecule. It uses dots to represent electrons and lines to show chemical bonds. For OF2, this diagram helps visualize how oxygen and fluorine atoms share electrons to form a stable molecule. (Lewis dot structure, molecular geometry)
Steps to Draw the Lewis Dot Diagram for OF2
Step 1: Determine the Total Number of Valence Electrons
Oxygen (O) has 6 valence electrons, and each fluorine (F) atom has 7 valence electrons. Since OF2 has one oxygen and two fluorine atoms:
- Oxygen: 6 electrons
- Fluorine (2 atoms): 2 × 7 = 14 electrons
- Total valence electrons: 6 + 14 = 20 electrons
Step 2: Identify the Central Atom
In OF2, oxygen is the central atom because it is less electronegative than fluorine. This is unusual, as oxygen is typically more electronegative, but in this case, it serves as the central atom due to the molecule’s structure. (Central atom, electronegativity)
Step 3: Connect Atoms with Single Bonds
Draw single bonds between the oxygen atom and each fluorine atom. This uses up 4 electrons (2 bonds × 2 electrons per bond), leaving 16 electrons to be distributed as lone pairs.
Step 4: Distribute Remaining Electrons
Place the remaining electrons as lone pairs on the atoms, starting with the outer atoms (fluorine). Each fluorine atom will have 3 lone pairs (6 electrons each), and the oxygen atom will have 2 lone pairs (4 electrons). This satisfies the octet rule for all atoms. (Octet rule, lone pairs)
💡 Note: Oxygen in OF2 has a formal charge of +1, while each fluorine has a formal charge of -1. This is due to the unequal sharing of electrons.
Final Lewis Dot Diagram for OF2
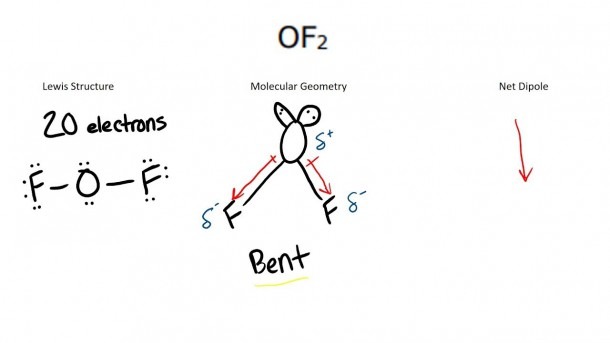
The completed Lewis dot diagram for OF2 shows:
- Oxygen as the central atom with 2 lone pairs.
- Two fluorine atoms, each connected to oxygen by a single bond and having 3 lone pairs.
This structure accurately represents the electron distribution in OF2. (Molecular structure, electron distribution)
Key Takeaways

- OF2 has a total of 20 valence electrons.
- Oxygen acts as the central atom despite being less electronegative in this case.
- The molecule follows the octet rule with lone pairs on both oxygen and fluorine atoms.
What is the molecular geometry of OF2?
+The molecular geometry of OF2 is bent due to the lone pairs on the oxygen atom, which cause electron repulsion.
Why does oxygen have a formal charge in OF2?
+Oxygen has a formal charge of +1 in OF2 because it shares its electrons unequally with the more electronegative fluorine atoms.
How does the Lewis dot diagram help predict OF2’s properties?
+The Lewis dot diagram shows OF2’s electron distribution, helping predict its reactivity, polarity, and bonding behavior.
Mastering the Lewis dot diagram for OF2 enhances your understanding of molecular structures and chemical bonding. By following these steps, you can accurately represent OF2’s electron arrangement and predict its properties. Whether for academic or professional purposes, this knowledge is invaluable in chemistry. (Chemical bonding, molecular structure)



