lewis structure of scn
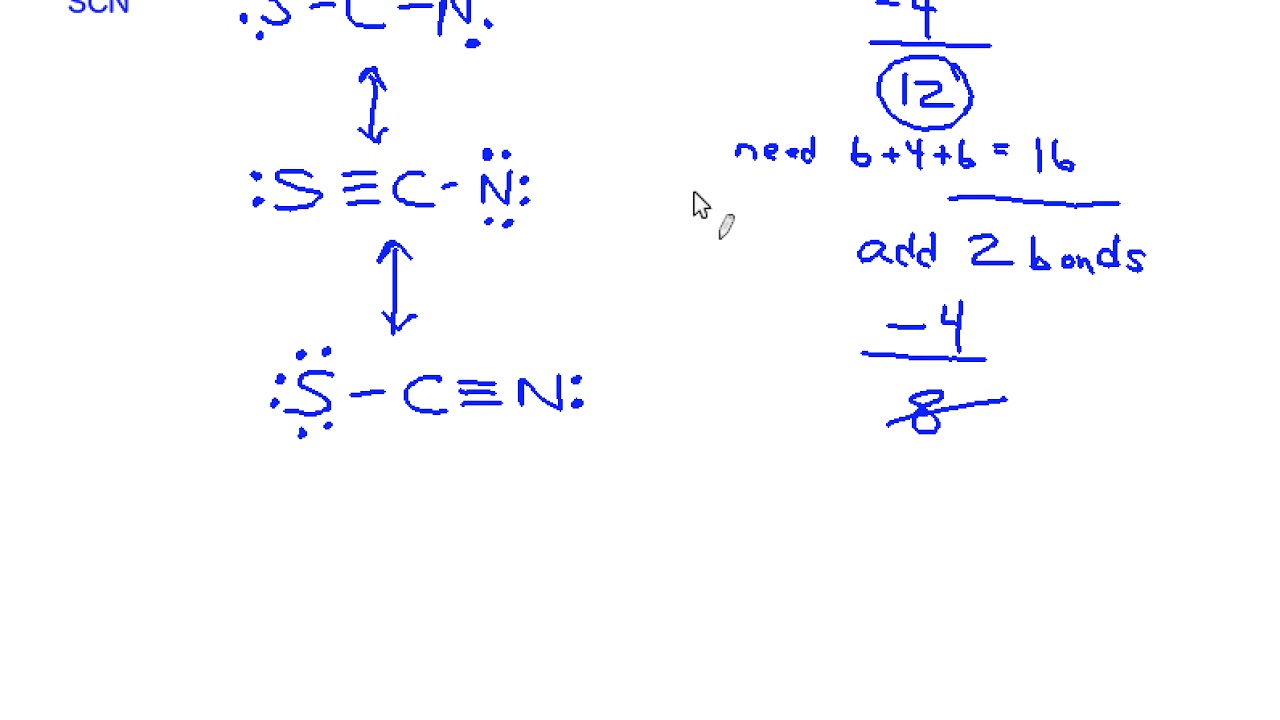
Understanding the Lewis structure of SCN (thiocyanate ion) is essential for students and professionals in chemistry, particularly in fields like inorganic chemistry and chemical bonding. The SCN- ion is a polyatomic ion composed of sulfur, carbon, and nitrogen atoms. Its Lewis structure helps visualize electron distribution, bonding, and molecular geometry. This guide will walk you through the step-by-step process of drawing the Lewis structure of SCN-, explain its significance, and provide practical tips for mastering this concept. (lewis structure of scn, thiocyanate ion, chemical bonding)
What is the Lewis Structure of SCN-?

The Lewis structure of SCN- represents the arrangement of atoms and valence electrons in the thiocyanate ion. It consists of a sulfur atom (S) bonded to a carbon atom (C), which is in turn bonded to a nitrogen atom (N). The ion carries a negative charge, indicating an extra electron. Understanding this structure is crucial for predicting its reactivity and properties. (thiocyanate ion, lewis structure of scn, molecular geometry)
Key Steps to Draw the Lewis Structure of SCN-
- Step 1: Determine Total Valence Electrons - Sulfur has 6, carbon has 4, nitrogen has 5, and the negative charge adds 1, totaling 16 valence electrons. (valence electrons, lewis structure of scn)
- Step 2: Choose the Central Atom - Carbon is the central atom due to its ability to form multiple bonds. (central atom, thiocyanate ion)
- Step 3: Arrange Atoms and Form Bonds - Connect S to C and C to N with single bonds, using 6 electrons. (chemical bonding, lewis structure of scn)
- Step 4: Complete Octets - Distribute remaining electrons to complete octets, prioritizing nitrogen and sulfur. (octet rule, thiocyanate ion)
- Step 5: Check Formal Charges - Ensure the structure minimizes formal charges for stability. (formal charges, lewis structure of scn)
💡 Note: The negative charge in SCN- is often delocalized between nitrogen and sulfur, depending on the resonance structure. (resonance structures, thiocyanate ion)
Resonance Structures of SCN-
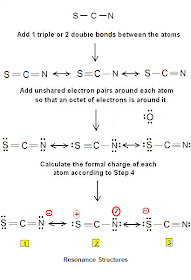
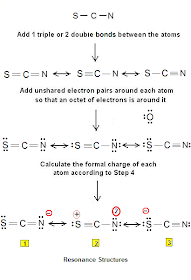
The SCN- ion exhibits resonance, meaning it has multiple valid Lewis structures. These structures differ in the placement of double bonds and the negative charge. Resonance stabilizes the ion and explains its unique chemical behavior. (resonance structures, lewis structure of scn, thiocyanate ion)
| Resonance Structure | Charge Distribution |
|---|---|
| S=C-N- | Negative charge on N |
| S--C≡N | Negative charge on S |
Practical Applications of SCN-
The thiocyanate ion is widely used in analytical chemistry, particularly in the qualitative test for iron(III) ions. Its Lewis structure helps chemists understand its role in coordination complexes and reactions. (thiocyanate ion, analytical chemistry, lewis structure of scn)
Checklist for Drawing the Lewis Structure of SCN-
- Count total valence electrons (16)
- Place carbon as the central atom
- Form single bonds between atoms
- Distribute remaining electrons to complete octets
- Check formal charges for stability
- Consider resonance structures (lewis structure of scn, thiocyanate ion)
Mastering the Lewis structure of SCN- is a fundamental skill in chemistry. By following the steps outlined above, you can accurately represent the ion’s bonding and electron distribution. Understanding resonance structures further enhances your grasp of its chemical behavior. Whether for academic purposes or practical applications, this knowledge is invaluable. (lewis structure of scn, thiocyanate ion, chemical bonding)
What is the central atom in the SCN- Lewis structure?
+
Carbon © is the central atom in the SCN- Lewis structure due to its ability to form multiple bonds. (central atom, lewis structure of scn)
How many valence electrons are in the SCN- ion?
+
The SCN- ion has 16 valence electrons, calculated by adding the electrons from sulfur (6), carbon (4), nitrogen (5), and the negative charge (1). (valence electrons, lewis structure of scn)
Why does SCN- have resonance structures?
+
SCN- has resonance structures because the double bond and negative charge can be delocalized between nitrogen and sulfur, providing stability. (resonance structures, thiocyanate ion)


