Understanding the Mass of Water: Essential Facts & Insights
Water is one of the most abundant substances on Earth, yet its mass and properties are often misunderstood. Whether you’re a student, a scientist, or simply curious, understanding the mass of water is crucial for various applications, from chemistry to everyday life. This post delves into essential facts and insights about the mass of water, tailored for both informational and commercial audiences. (mass of water, water density, water properties)
What is the Mass of Water?

The mass of water is a fundamental concept in science, referring to the amount of matter in a given volume of water. Water’s mass is typically measured in grams (g) or kilograms (kg). One of the most critical aspects to understand is that 1 liter (L) of water at 4°C weighs exactly 1 kilogram (kg). This relationship is based on water’s density, which is 1 gram per cubic centimeter (g/cm³) at this temperature. (water mass calculation, water weight)
How Density Affects Water’s Mass

Density is the mass per unit volume of a substance. Water’s density varies with temperature, which directly impacts its mass. For instance:
- At 0°C (freezing point), water’s density is approximately 0.9998 g/cm³.
- At 100°C (boiling point), it decreases to 0.9584 g/cm³.
This variation is due to the molecular structure of water, which expands as it heats up. Understanding these changes is vital for industries like food processing, pharmaceuticals, and environmental science. (water density, temperature effects on water)
| Temperature (°C) | Density (g/cm³) |
|---|---|
| 0 | 0.9998 |
| 4 | 1.0000 |
| 20 | 0.9982 |
| 100 | 0.9584 |

Practical Applications of Water’s Mass

For informational-intent readers, knowing water’s mass helps in scientific experiments, cooking, and understanding natural phenomena like rainfall and ocean currents. For commercial-intent visitors, this knowledge is essential for industries such as agriculture, manufacturing, and water treatment. (water applications, industrial uses of water)
Key Uses in Daily Life
- Cooking: Precise measurements of water mass ensure consistent recipes.
- Health: Monitoring water intake by mass helps in hydration tracking.
- Gardening: Understanding water’s mass aids in efficient irrigation.
💡 Note: Always account for temperature when measuring water mass for precise results.
How to Calculate Water’s Mass

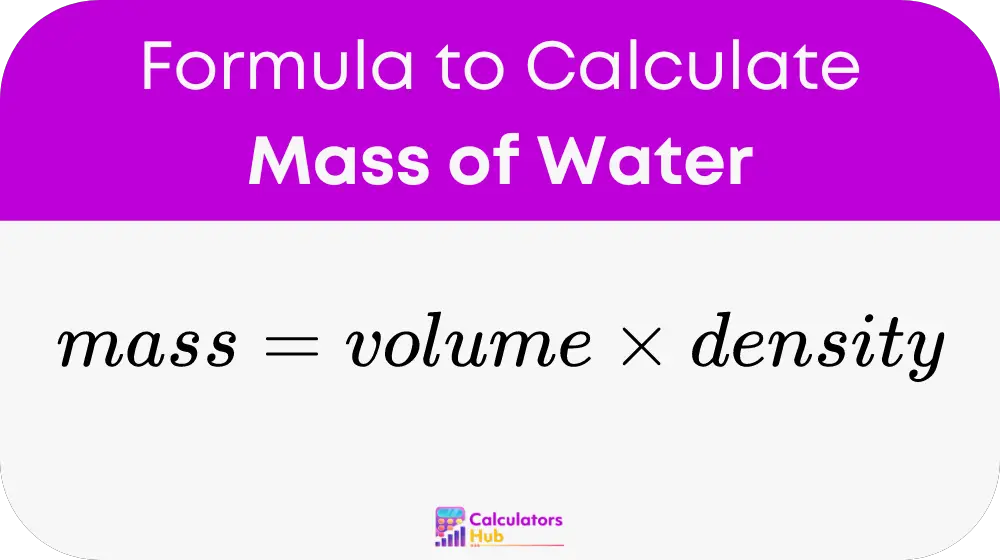
Calculating water’s mass is straightforward using the formula:
Mass (g) = Volume (mL) × Density (g/mL)
For example, 500 mL of water at 4°C would weigh:
500 mL × 1 g/mL = 500 g.
This calculation is invaluable in laboratories, educational settings, and industrial processes. (water mass calculation, volume to mass conversion)
Commercial Tools for Measuring Water Mass

For businesses, accurate measurement of water mass is critical. Tools like digital scales, hydrometers, and density meters are widely used. Investing in high-quality equipment ensures precision in industries such as beverage production, chemical manufacturing, and wastewater management. (water measurement tools, industrial scales)
Checklist for Accurate Water Mass Measurement
- Calibrate tools regularly to ensure accuracy.
- Account for temperature when measuring density.
- Use appropriate units (g, kg, or mL) based on application.
Wrapping Up
Understanding the mass of water is essential for both scientific curiosity and practical applications. From its density variations with temperature to its role in industries, water’s mass is a cornerstone of knowledge. Whether you’re a student, a professional, or a hobbyist, grasping these concepts will enhance your understanding of this vital resource. (water mass, water density, practical applications)
What is the mass of 1 liter of water?
+At 4°C, 1 liter of water weighs exactly 1 kilogram (kg).
How does temperature affect water’s mass?
+Water’s density changes with temperature, affecting its mass per unit volume. It is highest at 4°C and decreases as temperature rises or falls.
Why is water’s mass important in industries?
+Accurate measurement of water’s mass ensures quality control, efficiency, and safety in industries like food production, pharmaceuticals, and manufacturing.



