Methane Intermolecular Forces: Understanding the Basics

Methane, a simple yet fascinating molecule, plays a crucial role in various scientific and industrial applications. Understanding its intermolecular forces is essential for grasping its behavior in different environments. In this post, we’ll delve into the methane intermolecular forces, exploring their types, significance, and real-world implications. Whether you’re a student, researcher, or industry professional, this guide will provide valuable insights tailored to your needs.
What Are Intermolecular Forces?

Intermolecular forces (IMFs) are the attractions between molecules that dictate their physical properties, such as boiling point, melting point, and solubility. Unlike intramolecular forces (bonds within a molecule), IMFs act between separate molecules. Methane (CH₄), being a nonpolar molecule, exhibits specific types of IMFs that influence its behavior.
Types of Intermolecular Forces in Methane
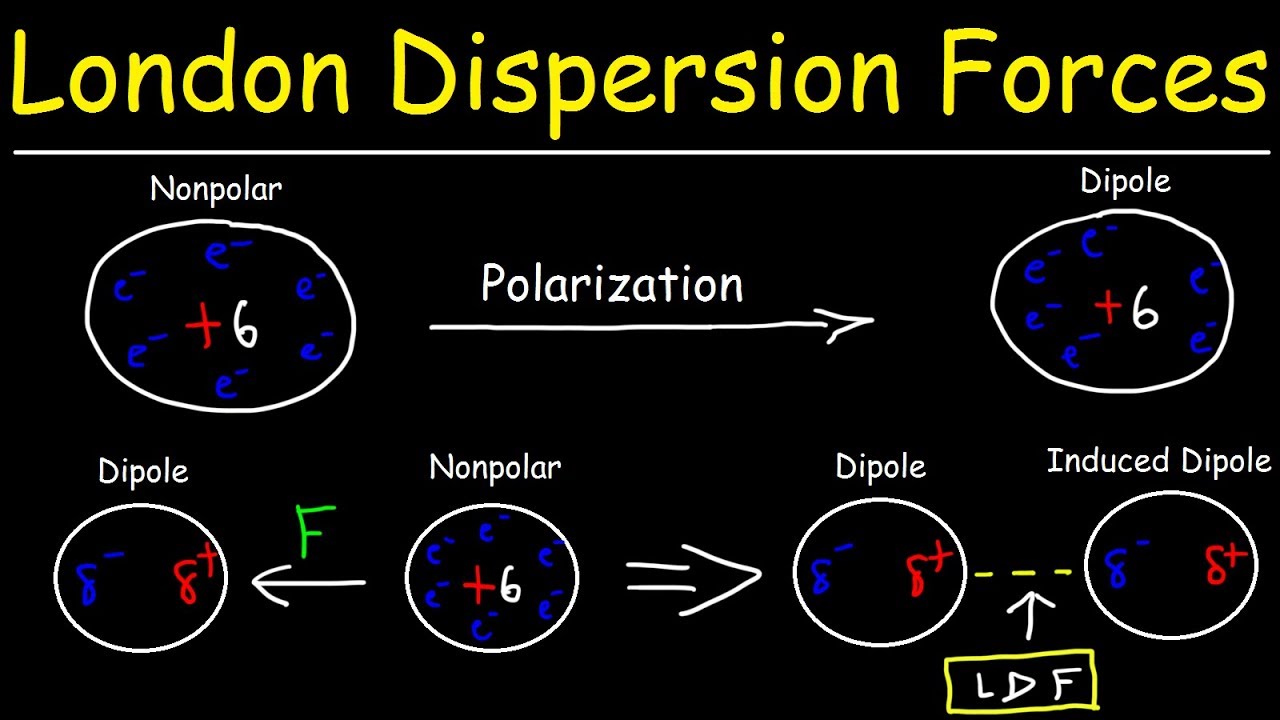
Methane primarily experiences London dispersion forces (LDFs), a type of IMF that arises due to temporary fluctuations in electron distribution. These forces are weak but universal, affecting all molecules, including nonpolar ones like methane.
| Type of IMF | Description | Strength |
|---|---|---|
| London Dispersion Forces | Temporary dipoles caused by electron movement | Weak |

Why Are Methane’s Intermolecular Forces Important?
Understanding methane’s IMFs is crucial for several reasons:
- Industrial Applications: Methane is a key component in natural gas and serves as a feedstock for chemicals and fuels.
- Environmental Impact: Methane is a potent greenhouse gas, and its behavior in the atmosphere is influenced by IMFs.
- Scientific Research: Studying methane’s IMFs provides insights into molecular interactions and material properties.
💡 Note: Methane’s weak IMFs explain its low boiling point (-161.5°C) and gaseous state at room temperature.
Comparing Methane’s IMFs to Other Molecules

To better understand methane’s IMFs, let’s compare them to other molecules:
- Water (H₂O): Exhibits strong hydrogen bonding, resulting in higher boiling and melting points.
- Ethanol (C₂H₅OH): Contains both hydrogen bonding and LDFs, making it more polar than methane.
- Carbon Dioxide (CO₂): A nonpolar molecule like methane but with stronger LDFs due to its larger size.
Practical Implications of Methane’s IMFs

For commercial-intent visitors, methane’s IMFs have direct applications in:
- Natural Gas Storage: Understanding IMFs helps optimize storage conditions.
- Chemical Manufacturing: Methane’s reactivity and behavior in reactions are influenced by its IMFs.
- Climate Science: Mitigating methane emissions requires knowledge of its atmospheric interactions.
Key Takeaways
- Methane’s intermolecular forces are primarily London dispersion forces.
- These forces are weak but universal, dictating methane’s physical properties.
- IMFs play a vital role in methane’s industrial, environmental, and scientific applications.
What are the main intermolecular forces in methane?
+Methane’s primary intermolecular force is London dispersion forces (LDFs), which arise from temporary dipoles.
Why is methane a gas at room temperature?
+Methane’s weak IMFs result in low boiling and melting points, keeping it in a gaseous state at room temperature.
How do methane’s IMFs compare to water’s?
+Water exhibits strong hydrogen bonding, while methane only has weak London dispersion forces.
Methane intermolecular forces,London dispersion forces,industrial applications of methane,greenhouse gases,molecular interactions,