NH3 Bonding Angle: Understanding Ammonia's Unique Geometry

Ammonia (NH₃) is a fundamental molecule in chemistry, known for its unique pyramidal geometry and significant role in various industries. The NH₃ bonding angle, approximately 107.3°, is a critical aspect of its structure, influenced by the arrangement of atoms and electron pairs. Understanding this angle is essential for grasping ammonia’s chemical behavior, applications, and its role in fields like agriculture, cleaning, and refrigeration. This blog explores the factors determining the NH₃ bonding angle, its implications, and why it deviates from the ideal tetrahedral angle of 109.5°.
What Determines the NH₃ Bonding Angle?
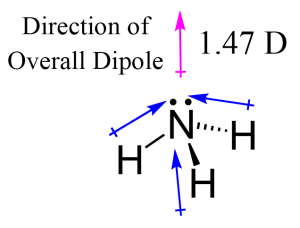
The NH₃ bonding angle is primarily influenced by two factors: the repulsion between electron pairs and the molecular geometry. Ammonia consists of one nitrogen atom bonded to three hydrogen atoms, with one lone pair of electrons on the nitrogen.
- Electron Pair Repulsion: According to the Valence Shell Electron Pair Repulsion (VSEPR) theory, electron pairs repel each other to maximize distance. In NH₃, the lone pair exerts greater repulsion than the bonding pairs, compressing the H-N-H bond angle to 107.3°.
- Molecular Geometry: The presence of a lone pair distorts the ideal tetrahedral geometry, resulting in a trigonal pyramidal shape.
📌 Note: The lone pair in NH₃ plays a crucial role in reducing the bond angle, making it smaller than the ideal 109.5°.
Why Is the NH₃ Bonding Angle Important?
The NH₃ bonding angle is not just a theoretical concept; it has practical implications in chemistry and industry.
- Chemical Reactivity: The lone pair on nitrogen makes NH₃ a strong nucleophile, essential in organic synthesis and biological processes.
- Industrial Applications: Ammonia’s geometry influences its role in fertilizers, cleaning agents, and refrigeration systems.
- Environmental Impact: Understanding NH₃ bonding helps in studying its role in atmospheric chemistry and pollution.
Comparing NH₃ Bonding Angle to Other Molecules

To better understand the NH₃ bonding angle, it’s helpful to compare it with similar molecules.
| Molecule | Bond Angle | Geometry |
|---|---|---|
| NH₃ (Ammonia) | 107.3° | Trigonal Pyramidal |
| CH₄ (Methane) | 109.5° | Tetrahedral |
| H₂O (Water) | 104.5° | Bent |

📌 Note: Methane (CH₄) has no lone pairs, resulting in an ideal tetrahedral angle, while water (H₂O) has two lone pairs, further reducing its bond angle.
Key Takeaways: NH₃ Bonding Angle Checklist

- Bond Angle: 107.3° due to lone pair repulsion.
- Geometry: Trigonal pyramidal shape.
- Applications: Essential in fertilizers, cleaning, and refrigeration.
- Comparison: Smaller angle than CH₄, larger than H₂O.
The NH₃ bonding angle is a fascinating example of how molecular structure dictates chemical properties. By understanding this angle, we gain insights into ammonia’s reactivity, geometry, and applications. Whether you’re a student, researcher, or industry professional, grasping this concept is key to mastering chemistry (molecular geometry,VSEPR theory,chemical bonding,ammonia structure,industrial chemistry).
What causes the NH₃ bonding angle to be 107.3°?
+
The lone pair on the nitrogen atom exerts greater repulsion than the bonding pairs, compressing the H-N-H bond angle from the ideal 109.5° to 107.3°.
How does the NH₃ bonding angle affect its chemical properties?
+
The presence of a lone pair makes NH₃ a strong nucleophile, influencing its reactivity in chemical reactions and industrial applications.
Why is NH₃’s bond angle different from methane (CH₄)?
+
Methane has no lone pairs, resulting in an ideal tetrahedral angle of 109.5°, while NH₃’s lone pair reduces its bond angle to 107.3°.



