Phosphorus Valence Electrons: Unlocking the Chemistry Secrets

Phosphorus, a vital element in chemistry, plays a crucial role in various industries, from agriculture to pharmaceuticals. Understanding its valence electrons is key to unlocking its chemical behavior and applications. In this post, we’ll explore phosphorus valence electrons, their significance, and how they influence its reactivity and bonding. Whether you’re a chemistry enthusiast or a professional, this guide will provide valuable insights into the world of phosphorus.
What Are Phosphorus Valence Electrons?
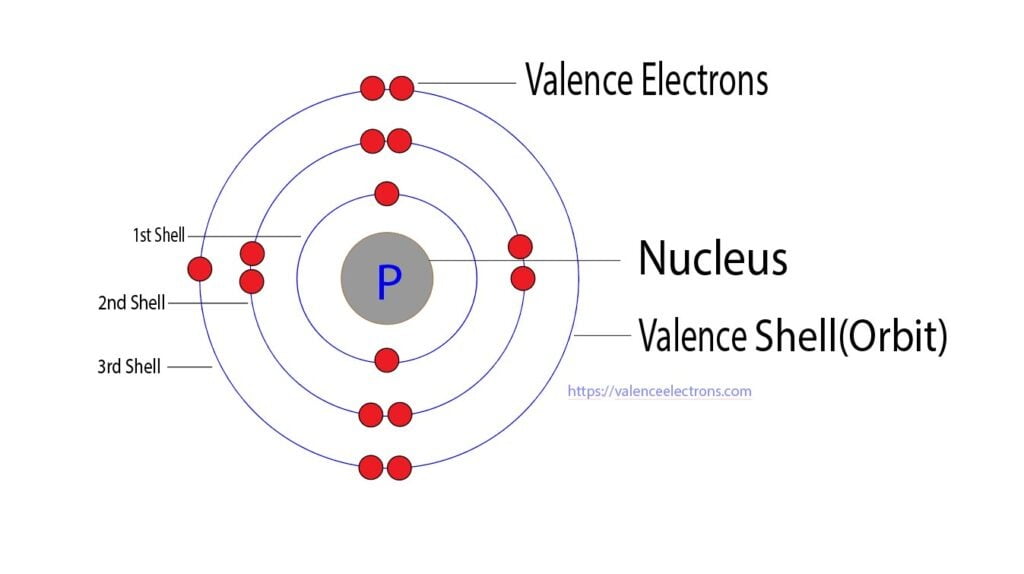
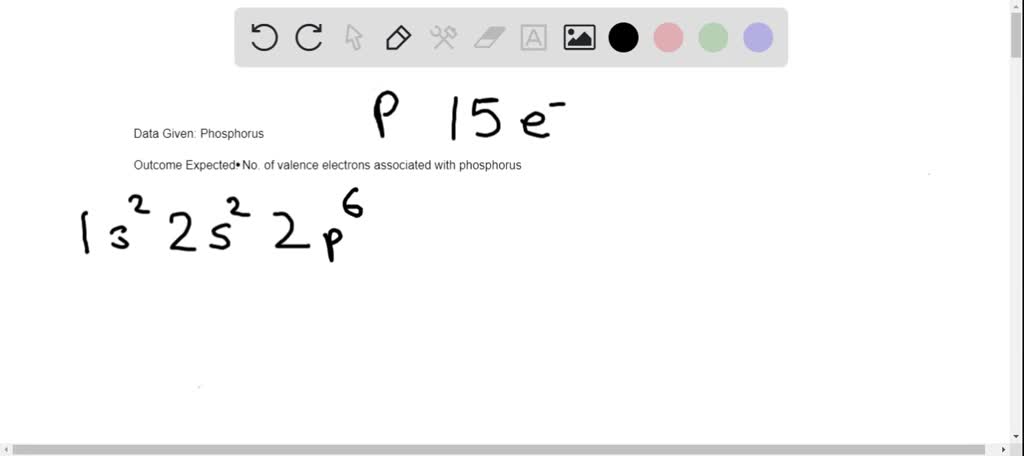
Phosphorus, with the atomic number 15, has 5 valence electrons in its outermost shell. These electrons determine how phosphorus interacts with other elements, forming compounds essential for life and industry. The electron configuration of phosphorus is 1s² 2s² 2p⁶ 3s² 3p³, with the 3s² 3p³ electrons being the valence electrons.
📌 Note: Valence electrons are the electrons in the outermost shell of an atom that participate in chemical bonding.
Why Are Phosphorus Valence Electrons Important?

The 5 valence electrons of phosphorus allow it to form multiple bonds, making it highly versatile in chemical reactions. This property is crucial in:
- Biological systems: Phosphorus is a key component of DNA, RNA, and ATP.
- Agriculture: Phosphate fertilizers enhance crop growth.
- Industry: Phosphorus compounds are used in detergents, pesticides, and electronics.
Bonding Behavior of Phosphorus
Phosphorus can form covalent bonds, ionic bonds, and even coordinate bonds due to its valence electrons. For example:
- Phosphoric acid (H₃PO₄): Forms by sharing its valence electrons with oxygen and hydrogen.
- Phosphorus pentachloride (PCl₅): Exhibits expanded octet behavior, utilizing d-orbitals for bonding.
| Compound | Bond Type | Application |
|---|---|---|
| H₃PO₄ | Covalent | Food additives, rust removal |
| PCl₅ | Covalent | Chlorinating agent |

How to Determine Phosphorus Valence Electrons

To identify phosphorus valence electrons:
1. Locate its position on the periodic table: Phosphorus is in Group 15 (VA).
2. Check its electron configuration: The outermost shell (3s² 3p³) contains 5 electrons.
✨ Note: Group number in the periodic table (for main-group elements) indicates the number of valence electrons.
Applications of Phosphorus Compounds

Understanding phosphorus valence electrons is essential for optimizing its applications:
- Fertilizers: Enhances plant growth by providing essential phosphate ions.
- Detergents: Phosphates remove hard water minerals.
- Medicine: Phosphorus-based compounds are used in drugs and medical imaging.
Key Takeaways
- Phosphorus has 5 valence electrons in its outermost shell.
- Its valence electrons enable diverse bonding and reactivity.
- Phosphorus compounds are vital in biology, agriculture, and industry.
How many valence electrons does phosphorus have?
+Phosphorus has 5 valence electrons in its outermost shell.
What is the electron configuration of phosphorus?
+The electron configuration of phosphorus is 1s² 2s² 2p⁶ 3s² 3p³.
Why is phosphorus important in agriculture?
+Phosphorus is essential for plant growth as it is a key component of fertilizers, providing phosphate ions that enhance root development and overall health.
Phosphorus valence electrons,phosphorus compounds,chemical bonding,periodic table,fertilizers,detergents,biological systems,phosphoric acid,phosphorus pentachloride.