Potassium Bohr Diagram: A Simple Visual Guide

Understanding the Potassium Bohr Diagram is essential for anyone studying chemistry or atomic structure. This simple visual guide breaks down the electron configuration of potassium, making it easier to grasp its atomic arrangement. Whether you're a student, educator, or just curious about chemistry, this guide will help you visualize potassium’s electrons in a clear and concise manner. From its atomic number to electron shell distribution, we’ll cover everything you need to know to master the Potassium Bohr Diagram, potassium electron configuration, and atomic structure of potassium.
What is a Bohr Diagram?
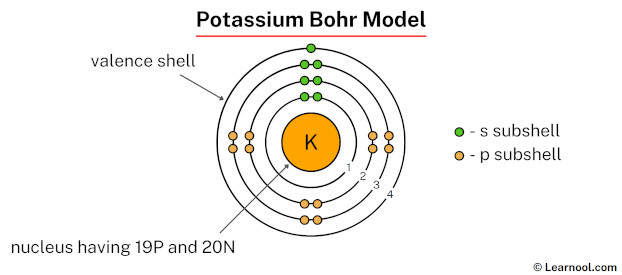
A Bohr Diagram is a visual representation of an atom’s electron configuration, named after physicist Niels Bohr. It shows the arrangement of electrons in different energy levels or shells around the nucleus. For potassium, a Potassium Bohr Diagram illustrates its 19 electrons distributed across shells, providing insights into its chemical behavior and reactivity. Understanding this diagram is crucial for topics like potassium electron configuration and atomic structure of potassium.
Potassium’s Atomic Structure

Potassium, with the atomic number 19, has 19 protons and 19 electrons. Its electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹. In a Potassium Bohr Diagram, these electrons are arranged in four shells: the first shell holds 2 electrons, the second shell holds 8, the third shell holds 8, and the fourth shell holds 1. This outer electron in the 4s orbital is what makes potassium highly reactive, a key aspect of its atomic structure of potassium.
How to Draw a Potassium Bohr Diagram

Drawing a Potassium Bohr Diagram is straightforward. Follow these steps:
- Step 1: Write the atomic number (19) in the center to represent the nucleus.
- Step 2: Draw the first shell and place 2 electrons in it.
- Step 3: Add the second shell and place 8 electrons in it.
- Step 4: Draw the third shell and place 8 electrons in it.
- Step 5: Finally, add the fourth shell and place 1 electron in it.
📌 Note: Ensure each shell is drawn proportionally to represent the atom’s structure accurately.
Importance of the Potassium Bohr Diagram

The Potassium Bohr Diagram is vital for understanding potassium’s chemical properties. Its single valence electron in the 4s orbital explains why potassium is highly reactive and readily loses this electron to form a +1 ion. This knowledge is essential for studying potassium electron configuration, atomic structure of potassium, and its role in chemical reactions.
Key Takeaways Checklist
- Potassium has 19 electrons, arranged in 4 shells.
- The electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹.
- The Bohr Diagram shows 2 electrons in the first shell, 8 in the second, 8 in the third, and 1 in the fourth.
- Potassium’s reactivity is due to its single valence electron in the 4s orbital.
In summary, the Potassium Bohr Diagram is a powerful tool for visualizing the atomic structure of potassium. By understanding its electron configuration and shell distribution, you can better grasp potassium’s chemical behavior. Whether you’re studying potassium electron configuration or exploring the atomic structure of potassium, this guide provides a clear and concise overview to enhance your knowledge, Bohr Diagram, potassium electron configuration, atomic structure of potassium.
What is the electron configuration of potassium?
+
The electron configuration of potassium is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹.
Why is potassium highly reactive?
+
Potassium is highly reactive due to its single valence electron in the 4s orbital, which it readily loses to form a +1 ion.
How many shells are in the Potassium Bohr Diagram?
+
The Potassium Bohr Diagram has 4 shells, each containing 2, 8, 8, and 1 electrons, respectively.



