Potassium Electron Dot Diagram: Quick Visual Guide

Understanding the potassium electron dot diagram is essential for grasping the element’s electron configuration and chemical bonding behavior. Potassium, with its atomic number 19, plays a crucial role in various scientific and industrial applications. This quick visual guide will walk you through creating and interpreting the electron dot diagram for potassium, making it easier to understand its valence electrons and reactivity.
What is a Potassium Electron Dot Diagram?

A potassium electron dot diagram is a visual representation of potassium’s valence electrons. Potassium (K) has 19 electrons, with one valence electron in its outermost shell. This diagram uses dots around the element’s symbol to illustrate these electrons, providing a clear picture of its bonding capabilities.
How to Draw the Potassium Electron Dot Diagram

Drawing the electron dot diagram for potassium is straightforward. Follow these steps:
- Write the Symbol: Start by writing the chemical symbol for potassium, K.
- Add Dots for Valence Electrons: Since potassium has one valence electron, place a single dot around the symbol.
📌 Note: The dot is placed on any side of the symbol, but typically, it’s shown on the right for simplicity.
Why is the Potassium Electron Dot Diagram Important?

The potassium electron dot diagram is vital for understanding:
- Chemical Bonding: It shows how potassium forms ionic bonds by losing its single valence electron.
- Reactivity: Highlights its high reactivity due to the ease of losing one electron.
- Periodic Trends: Illustrates potassium’s position in Group 1 of the periodic table as an alkali metal.
Key Takeaways
- Potassium has one valence electron, represented by a single dot in its electron dot diagram.
- The diagram simplifies understanding potassium’s chemical behavior and bonding.
- It’s a valuable tool for students and professionals in chemistry and related fields.
To summarize, mastering the potassium electron dot diagram enhances your understanding of potassium’s properties and its role in chemical reactions. Whether for academic purposes or practical applications, this visual guide is an indispensable resource.
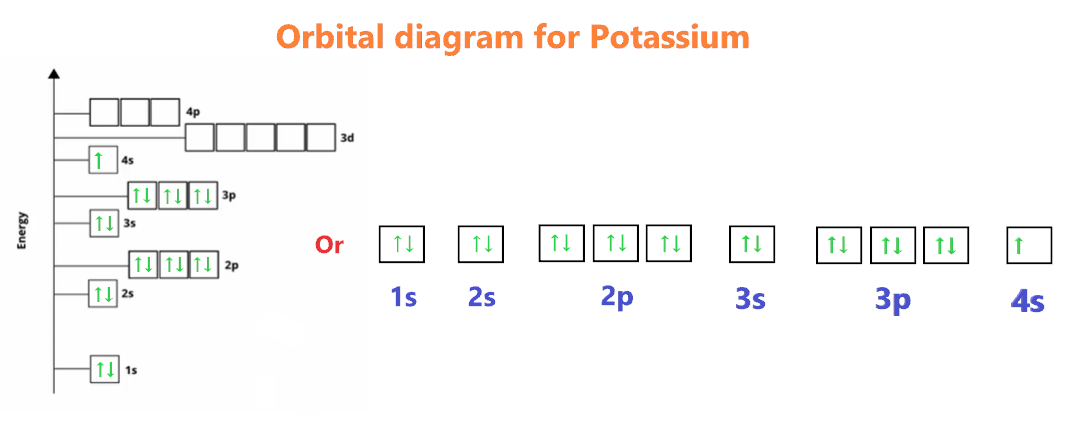
What is the electron configuration of potassium?
+Potassium's electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹, with one electron in the outermost 4s orbital.
How does potassium form bonds?
+Potassium forms ionic bonds by losing its single valence electron to achieve a stable electron configuration.
Why is potassium highly reactive?
+Potassium is highly reactive due to its single valence electron, which is easily lost in chemical reactions.
(electron dot diagram, potassium electron configuration, chemical bonding, valence electrons, periodic table)



