Potassium Full Electron Configuration: Simplified Guide

Understanding the potassium full electron configuration is essential for students, chemists, and anyone interested in the periodic table. Potassium, with its atomic number 19, plays a crucial role in biological processes and chemical reactions. This guide simplifies the electron configuration of potassium, making it accessible for both informational and commercial purposes. Whether you're studying for an exam or looking to purchase potassium-related products, this post has you in mind.
What is Potassium Full Electron Configuration?

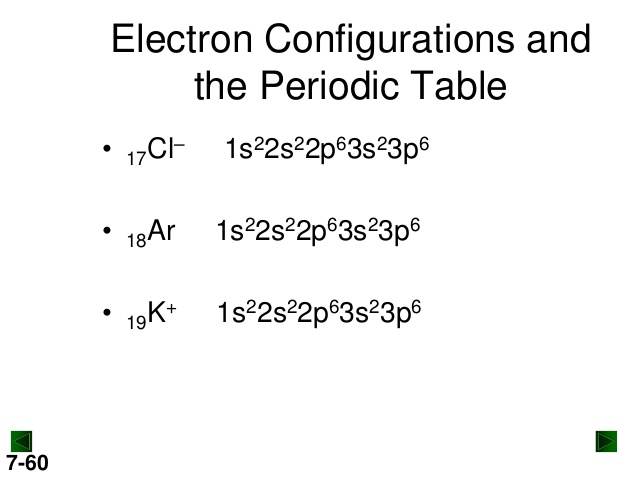
Potassium (K) has an atomic number of 19, meaning it has 19 electrons. The full electron configuration of potassium is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹. This configuration shows how electrons are distributed in potassium's energy levels and orbitals. Understanding this distribution is key to grasping potassium's chemical behavior, such as its reactivity and bonding patterns. For those looking to buy potassium compounds, knowing its electron configuration can help in selecting the right product for specific applications, (potassium compounds, chemical reactions, periodic table)
How to Write Potassium's Electron Configuration

Writing the electron configuration of potassium involves following the Aufbau principle, Pauli exclusion principle, and Hund's rule. Here’s a step-by-step breakdown:
- Step 1: Start with the lowest energy level (1s) and fill it with 2 electrons: 1s².
- Step 2: Move to the next level (2s) and fill it with 2 electrons: 2s².
- Step 3: Fill the 2p orbital with 6 electrons: 2p⁶.
- Step 4: Continue with the 3s orbital, adding 2 electrons: 3s².
- Step 5: Fill the 3p orbital with 6 electrons: 3p⁶.
- Step 6: Finally, add the last electron to the 4s orbital: 4s¹.
📌 Note: The 4s¹ configuration explains potassium's high reactivity, as it easily loses this electron to achieve a stable noble gas configuration.
Simplified Electron Configuration Using Noble Gas Notation

For a quicker representation, use the noble gas notation. Potassium's configuration can be written as [Ar] 4s¹, where [Ar] represents the electron configuration of argon, the preceding noble gas. This shorthand is useful for saving time and focusing on the valence electrons, which are crucial for chemical bonding, (noble gas notation, valence electrons, chemical bonding)
| Full Configuration | Noble Gas Notation |
|---|---|
| 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ | [Ar] 4s¹ |
Why Potassium's Electron Configuration Matters
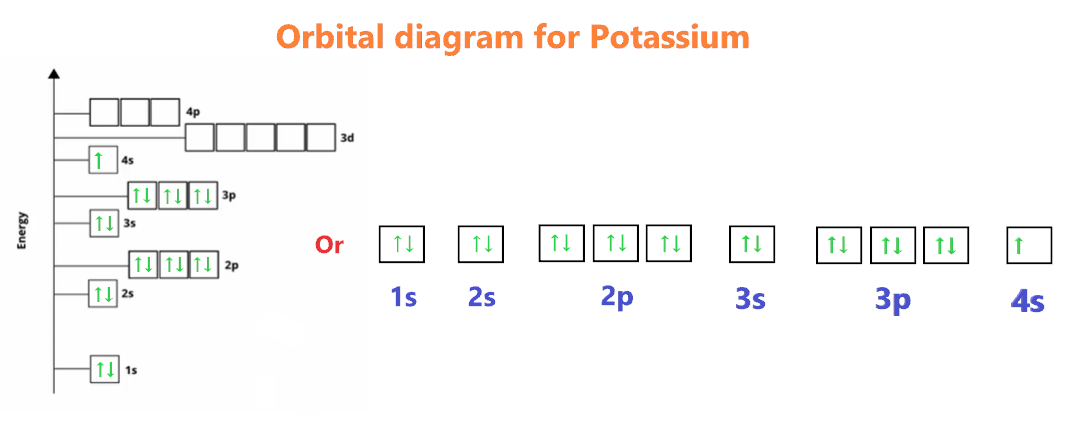
Potassium's electron configuration is vital for understanding its properties and applications. Its single valence electron in the 4s orbital makes it highly reactive, especially with elements like chlorine to form potassium chloride (KCl). This reactivity is why potassium is used in fertilizers, medicines, and even in some commercial products like potassium supplements. For buyers, knowing this ensures you select the right potassium-based product for your needs, (potassium chloride, fertilizers, potassium supplements)
Key Takeaways and Checklist
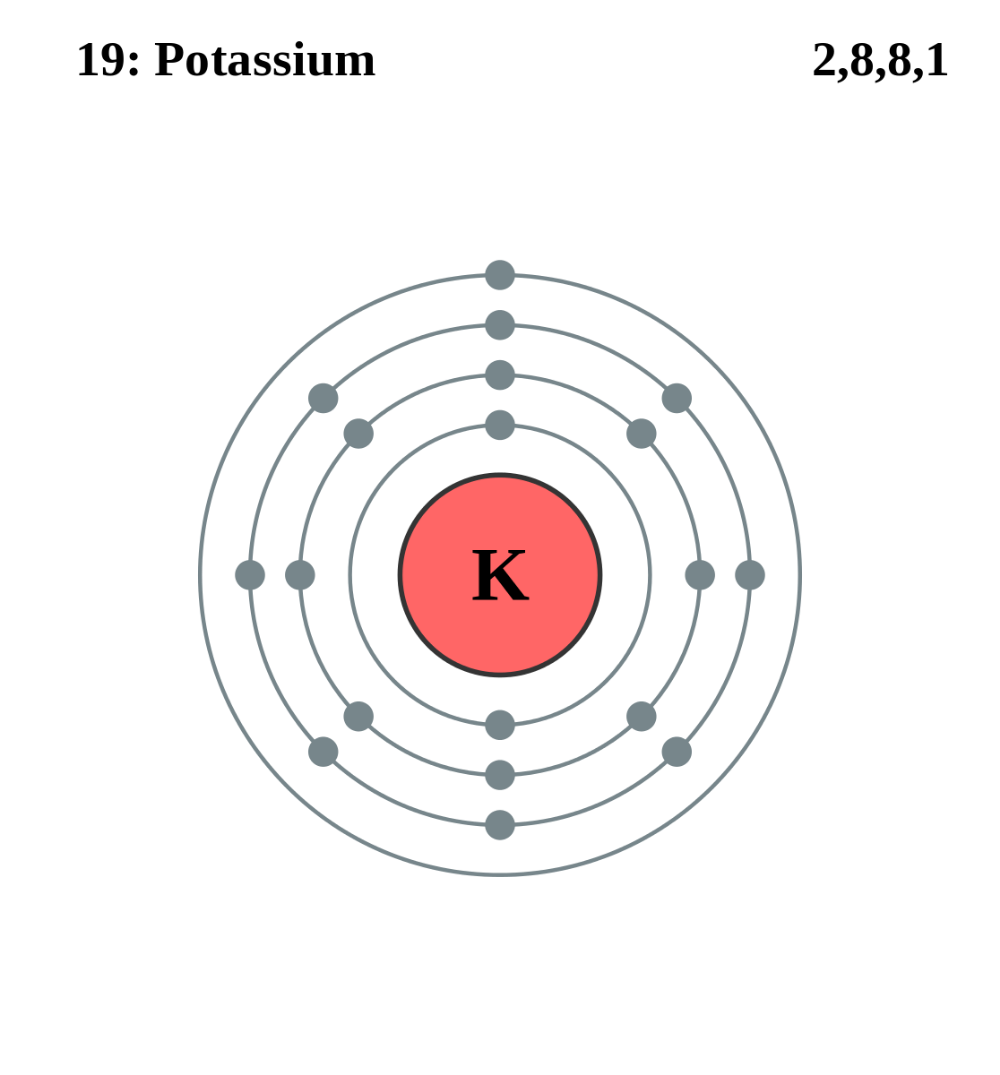
To summarize, here’s what you need to remember about potassium's electron configuration:
- Full configuration: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹
- Noble gas notation: [Ar] 4s¹
- Single valence electron in the 4s orbital
- High reactivity due to the loose hold on the valence electron
Checklist for Understanding Potassium's Electron Configuration:
- ✔ Learn the full and shorthand configurations
- ✔ Understand the role of the valence electron
- ✔ Apply knowledge to chemical reactions and applications
Mastering potassium's full electron configuration is a foundational step in chemistry. Whether you're a student, researcher, or consumer, this knowledge helps in understanding potassium's role in various fields. From its reactivity to its applications, potassium's electron configuration is a key to unlocking its potential. Use this guide to simplify your learning or purchasing decisions, ensuring you make informed choices in both academic and commercial contexts.
What is the full electron configuration of potassium?
+The full electron configuration of potassium is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹.
Why is potassium highly reactive?
+Potassium is highly reactive due to its single valence electron in the 4s orbital, which it easily loses to achieve a stable configuration.
What is the noble gas notation for potassium?
+The noble gas notation for potassium is [Ar] 4s¹.
Where is potassium used commercially?
+Potassium is used in fertilizers, medicines, and supplements due to its chemical properties and reactivity.