Potassium Lewis Dot Structure: Simple Visual Guide

Understanding the Potassium Lewis Dot Structure is essential for anyone studying chemistry, especially when it comes to grasping the electron configuration of elements. Potassium, a key alkali metal, plays a significant role in various chemical reactions and biological processes. By learning its Lewis dot structure, you can visualize its valence electrons and how it forms bonds with other elements. This guide provides a simple, step-by-step approach to drawing the Potassium Lewis Dot Structure, making it easy for both students and enthusiasts to master this concept. Whether you're preparing for an exam or simply curious about chemistry, this post will help you navigate the process effortlessly. (Potassium Lewis Dot Structure, Lewis Dot Structure, Electron Configuration)
What is the Potassium Lewis Dot Structure?

The Potassium Lewis Dot Structure represents the arrangement of valence electrons around a potassium atom. Potassium (K) has an atomic number of 19, with its electron configuration being 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹. This means it has one valence electron in its outermost shell. The Lewis dot structure simplifies this by placing a single dot around the symbol K, representing its lone valence electron. (Potassium Electron Configuration, Valence Electrons)
Step-by-Step Guide to Drawing the Potassium Lewis Dot Structure
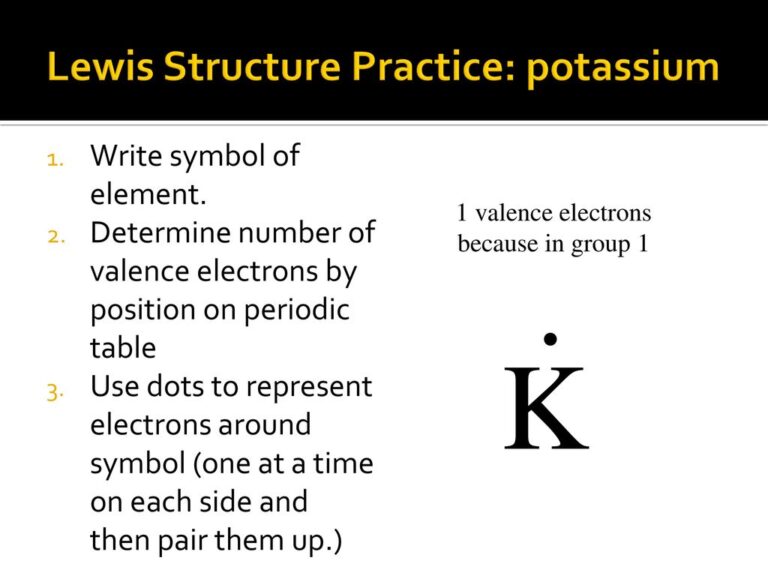
Step 1: Identify the Symbol and Valence Electrons
Start by writing the chemical symbol for potassium, K. Determine its valence electrons by looking at its electron configuration. Potassium has one valence electron in the 4s orbital. (Chemical Symbol, Valence Electrons)
Step 2: Place the Dot Around the Symbol
Draw a single dot around the symbol K to represent its valence electron. The dot should be placed on any side of the symbol, though conventionally, it’s placed to the right. (Lewis Dot Structure, Electron Representation)
📌 Note: Potassium tends to lose its single valence electron easily, forming a +1 ion (K⁺), which is why its Lewis dot structure is often simplified to just the symbol K without dots when considering its ionic form.
Step 3: Review and Confirm
Double-check that you’ve placed only one dot around the symbol K. This confirms the correct Lewis dot structure for potassium. (Lewis Dot Structure, Potassium Ion)
| Element | Symbol | Valence Electrons | Lewis Dot Structure |
|---|---|---|---|
| Potassium | K | 1 | K· |

Checklist for Drawing the Potassium Lewis Dot Structure
- Identify the chemical symbol (K)
- Determine the number of valence electrons (1)
- Place one dot around the symbol
- Review and confirm the structure
Mastering the Potassium Lewis Dot Structure is a fundamental step in understanding the basics of chemistry. By following this simple guide, you can easily visualize potassium’s valence electron and its role in chemical bonding. Remember, potassium’s single valence electron makes it highly reactive, often leading to the formation of the K⁺ ion. Use the checklist provided to ensure accuracy in your drawings. Whether for academic purposes or personal curiosity, this knowledge will serve as a solid foundation for exploring more complex chemical concepts. (Potassium Lewis Dot Structure, Chemical Bonding, K⁺ Ion)
Why does potassium have only one dot in its Lewis structure?
+
Potassium has only one valence electron in its outermost shell, which is represented by a single dot in its Lewis dot structure.
How does potassium form bonds with other elements?
+
Potassium tends to lose its single valence electron, forming a +1 ion (K⁺), which then bonds with negatively charged ions like chloride (Cl⁻) to form compounds such as potassium chloride (KCl).
What is the electron configuration of potassium?
+
The electron configuration of potassium is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹, with one electron in the 4s orbital.



