Is SCN Polar or Nonpolar? Quick Chemistry Insight.
Understanding the polarity of chemical compounds is crucial in chemistry, as it influences their properties and interactions. One common question that arises is, “Is SCN polar or nonpolar?” Thiocyanate (SCN⁻) is a fascinating ion with unique characteristics, making it an essential topic for students and chemistry enthusiasts alike. In this post, we’ll explore the polarity of SCN⁻, breaking down its molecular structure, electronegativity, and bond nature to provide a clear answer.
What is SCN⁻?

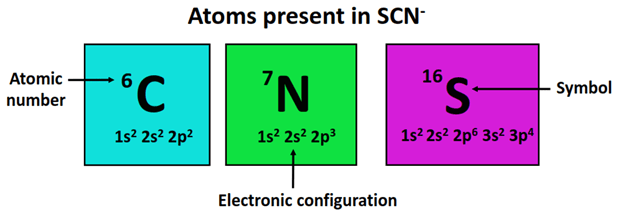
Thiocyanate (SCN⁻) is an anion composed of sulfur, carbon, and nitrogen atoms. It is commonly found in compounds like sodium thiocyanate (NaSCN) and potassium thiocyanate (KSCN). The ion’s structure is linear, with a triple bond between carbon and nitrogen (C≡N) and a single bond between sulfur and carbon (S−C).
Is SCN Polar or Nonpolar?

To determine if SCN⁻ is polar or nonpolar, we need to analyze its molecular geometry and electronegativity differences between atoms.
Molecular Geometry of SCN⁻
The SCN⁻ ion has a linear shape, similar to carbon dioxide (CO₂). In linear molecules, the bond dipoles often cancel each other out if the electronegativity differences are symmetrical.
Electronegativity Differences
- Sulfur (S): Electronegativity ≈ 2.58
- Carbon ©: Electronegativity ≈ 2.55
- Nitrogen (N): Electronegativity ≈ 3.04
The significant electronegativity difference between nitrogen and carbon (3.04 vs. 2.55) creates a polar C≡N bond. However, the S−C bond is nearly nonpolar due to the small electronegativity difference between sulfur and carbon.
Net Dipole Moment
Despite the polar C≡N bond, the linear geometry of SCN⁻ causes the bond dipoles to align in opposite directions, resulting in a net dipole moment of zero. This makes SCN⁻ a nonpolar molecule.
💡 Note: While SCN⁻ has polar bonds, its linear geometry cancels out the overall dipole moment, classifying it as nonpolar.
Why Polarity Matters

Understanding the polarity of SCN⁻ is essential for predicting its solubility, reactivity, and interactions with other molecules. Nonpolar molecules like SCN⁻ tend to dissolve in nonpolar solvents and exhibit specific chemical behaviors.
Key Takeaways

- SCN⁻ is nonpolar due to its linear geometry and canceling bond dipoles.
- The C≡N bond is polar, but the S−C bond is nearly nonpolar.
- Polarity influences solubility and chemical properties.
Checklist for Determining Polarity

- Step 1: Identify the molecular geometry.
- Step 2: Calculate electronegativity differences.
- Step 3: Determine bond polarity.
- Step 4: Assess net dipole moment.
What is the molecular geometry of SCN⁻?
+SCN⁻ has a linear molecular geometry, similar to CO₂.
Why is SCN⁻ considered nonpolar despite having polar bonds?
+The linear geometry of SCN⁻ causes the bond dipoles to cancel each other out, resulting in a net dipole moment of zero.
How does polarity affect SCN⁻’s solubility?
+As a nonpolar molecule, SCN⁻ is more soluble in nonpolar solvents than in polar ones.
In summary, SCN⁻ is nonpolar due to its linear structure and canceling bond dipoles. This insight is vital for understanding its chemical behavior and applications. Whether you’re a student or a professional, mastering such concepts enhances your chemistry knowledge. (chemical polarity, molecular geometry, electronegativity, bond dipole)



