Understanding HCN's Chemical Behavior: Valence Electrons Explained

Hydrogen cyanide (HCN) is a fascinating yet toxic compound that plays a significant role in various chemical processes. Understanding its behavior starts with grasping the role of valence electrons, which are the outermost electrons in an atom that participate in chemical bonding. In HCN, the valence electrons of hydrogen, carbon, and nitrogen dictate its reactivity, polarity, and overall chemical properties. This post delves into the importance of valence electrons in HCN, shedding light on its molecular structure and behavior. (HCN chemical properties, valence electrons in HCN, molecular structure of HCN)
What Are Valence Electrons?

Valence electrons are the electrons in the outermost energy level of an atom. They determine how an element will react and form bonds with other atoms. In HCN, hydrogen has 1 valence electron, carbon has 4, and nitrogen has 5. Together, these electrons form the basis of HCN’s linear molecular structure and its unique chemical behavior. (Valence electrons definition, electron configuration of HCN, chemical bonding in HCN)
| Atom | Valence Electrons |
|---|---|
| Hydrogen (H) | 1 |
| Carbon (C) | 4 |
| Nitrogen (N) | 5 |
How Valence Electrons Influence HCN’s Structure
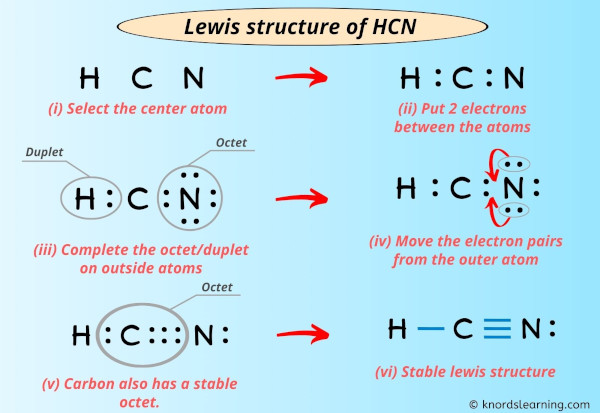
The arrangement of valence electrons in HCN results in a triple bond between carbon and nitrogen, with a single bond between hydrogen and carbon. This linear geometry gives HCN its polarity, as the electronegativity difference between nitrogen and hydrogen creates a net dipole moment. Understanding this structure is crucial for predicting HCN’s reactivity in chemical reactions. (HCN molecular geometry, polarity of HCN, triple bond in HCN)
📌 Note: HCN's linear structure and polarity make it a highly reactive molecule, often involved in industrial processes and biological systems.
The Role of Valence Electrons in HCN’s Reactivity

Valence electrons in HCN determine its ability to participate in reactions such as hydrolysis, polymerization, and coordination chemistry. For instance, the lone pair of electrons on nitrogen allows HCN to act as a ligand in coordination compounds. Additionally, its high reactivity with metals makes it a valuable reagent in synthesis reactions. (HCN reactivity, HCN in coordination chemistry, HCN synthesis)
- Hydrolysis: HCN reacts with water to form cyanide ions and hydronium ions.
- Polymerization: HCN can polymerize to form polycyanides under specific conditions.
- Coordination Chemistry: HCN acts as a ligand, forming complexes with transition metals.
Practical Applications of HCN

For commercial-intent visitors, HCN is widely used in industries such as pharmaceuticals, plastics, and mining. Its ability to form complexes with metals is exploited in gold and silver extraction processes. Additionally, HCN is a key intermediate in the production of acrylic fibers and resins. (Industrial uses of HCN, HCN in mining, HCN in pharmaceuticals)
📌 Note: Handling HCN requires strict safety measures due to its toxicity. Always follow industry guidelines for storage and use.
Key Takeaways: Understanding HCN’s Valence Electrons

- Valence Electrons: Determine HCN’s bonding and reactivity.
- Molecular Structure: Linear geometry with a triple bond between C and N.
- Polarity: Net dipole moment due to electronegativity differences.
- Reactivity: Involved in hydrolysis, polymerization, and coordination chemistry.
- Applications: Used in pharmaceuticals, mining, and plastics production.
(HCN key takeaways, HCN summary, HCN applications)
What are valence electrons in HCN?
+Valence electrons in HCN are the outermost electrons of hydrogen, carbon, and nitrogen that participate in chemical bonding, determining its structure and reactivity.
Why is HCN polar?
+HCN is polar due to the electronegativity difference between nitrogen and hydrogen, creating a net dipole moment in its linear structure.
What are the industrial uses of HCN?
+HCN is used in pharmaceuticals, plastics production, mining (gold and silver extraction), and as a key intermediate in chemical synthesis.
In summary, the valence electrons of HCN are fundamental to its chemical behavior, from its molecular structure to its reactivity and applications. Whether you’re studying chemistry or exploring industrial uses, understanding HCN’s valence electrons provides valuable insights into this versatile yet hazardous compound. (HCN chemical behavior, HCN insights, HCN hazardous compound)


