Weak Acid Strong Base Titration: A Comprehensive Guide
Weak Acid Strong Base Titration: Unlocking the Secrets of Acid-Base Reactions

Understanding weak acid strong base titration is crucial for anyone delving into the world of chemistry. This process allows us to determine the concentration of a weak acid by reacting it with a strong base of known concentration. It’s a fundamental technique used in various fields, from environmental analysis to pharmaceutical research. (acid-base titration, chemical analysis, laboratory techniques)
Understanding the Basics
Titration is a quantitative experimental technique used in chemistry to determine the concentration of a substance (the analyte) by reacting it with a known solution (the titrant). In weak acid strong base titration, the analyte is a weak acid, and the titrant is a strong base. (titration definition, analyte, titrant)
Weak acids only partially dissociate in water, meaning they don’t release all their hydrogen ions. Examples include acetic acid (found in vinegar) and citric acid (found in citrus fruits). Strong bases, on the other hand, fully dissociate in water, releasing a high concentration of hydroxide ions. Common examples are sodium hydroxide (NaOH) and potassium hydroxide (KOH). (weak acid definition, strong base definition, examples of weak acids, examples of strong bases)
The Titration Process: Step-by-Step
Preparation:
- Standardize the Strong Base: Before beginning, ensure your strong base solution is accurately concentrated. This often involves a separate titration using a primary standard acid.
- Measure the Weak Acid: Carefully measure a known volume of the weak acid solution into a flask.
Titration:
- Add Indicator: Choose an appropriate acid-base indicator that changes color at the desired pH range for your titration. Common indicators include phenolphthalein and bromothymol blue.
- Titrate: Slowly add the strong base solution from a burette into the weak acid flask, swirling constantly. The indicator will change color when the equivalence point is reached. This is the point where the moles of acid and base are stoichiometrically equivalent.
Data Analysis:
- Record Volume: Note the volume of strong base solution used to reach the equivalence point.
- Calculate Concentration: Use the known concentration of the strong base, the volume used, and the balanced chemical equation to calculate the concentration of the weak acid.
📝 Note: The choice of indicator is crucial for accurate results. Select an indicator with a pH range that encompasses the expected equivalence point.
(titration procedure, indicator selection, equivalence point, stoichiometry)Key Concepts and Considerations
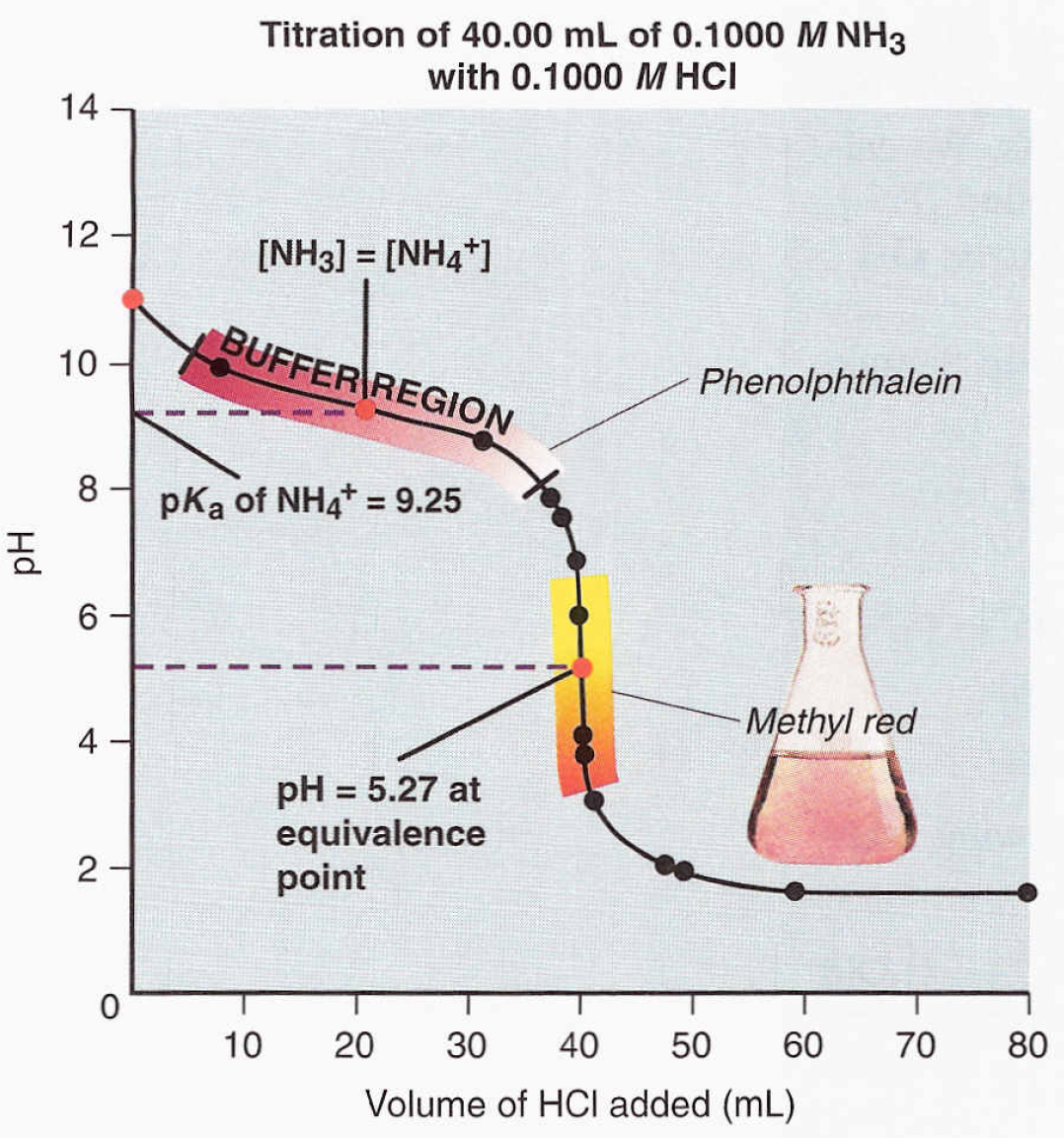
- pH Curve: Plotting pH against the volume of base added creates a titration curve. The curve’s shape provides valuable information about the strength of the acid and the progress of the reaction. (titration curve, pH curve analysis)
- Buffer Region: Near the equivalence point, the solution acts as a buffer, resisting drastic pH changes. This is due to the presence of both the weak acid and its conjugate base. (buffer solution, conjugate acid-base pairs)
- Half-Equivalence Point: At this point, half the weak acid has been neutralized. The pH at this point is equal to the pKa of the weak acid. (half-equivalence point, pKa)
Applications of Weak Acid Strong Base Titration
This versatile technique finds applications in numerous fields:
- Environmental Analysis: Determining the acidity of water samples, soil, and pollutants. (environmental monitoring, water quality analysis)
- Pharmaceutical Industry: Assessing the purity and concentration of drugs and active ingredients. (pharmaceutical analysis, drug development)
- Food and Beverage Industry: Measuring acidity levels in food products and beverages. (food quality control, beverage analysis)
Wrapping Up
Weak acid strong base titration is a powerful tool for quantifying the concentration of weak acids. By understanding the principles, procedures, and applications, you gain valuable insights into the world of acid-base chemistry. Remember, careful technique, accurate measurements, and appropriate indicator selection are key to obtaining reliable results. (acid-base chemistry, quantitative analysis, laboratory skills)
What is the difference between a weak acid and a strong acid?
+Weak acids only partially dissociate in water, while strong acids fully dissociate, releasing all their hydrogen ions.
Why is it important to standardize the strong base solution?
+Standardization ensures the concentration of the strong base is accurately known, allowing for precise calculations of the weak acid concentration.
How do I choose the right indicator for my titration?
+Select an indicator with a pH range that encompasses the expected equivalence point of your titration.



