BF3 Hybrid Orbitals: Structure & Bonding Explained

Understanding the concept of BF3 hybrid orbitals is crucial for anyone studying chemical bonding and molecular geometry. Boron trifluoride (BF3) is a fascinating molecule that exhibits sp² hybridization, a key principle in chemistry. This blog post will delve into the structure and bonding of BF3, explaining how hybrid orbitals contribute to its unique properties. Whether you're a student, researcher, or simply curious about molecular chemistry, this guide will provide clear insights into BF3 hybrid orbitals, their formation, and their role in chemical bonding. (BF3 hybridization, sp² hybridization, molecular geometry)
What Are BF3 Hybrid Orbitals?

BF3 hybrid orbitals are the result of hybridization, a process where atomic orbitals mix to form new hybrid orbitals with different energies and shapes. In BF3, boron’s atomic orbitals undergo sp² hybridization, combining one s orbital and two p orbitals. This results in three sp² hybrid orbitals arranged in a trigonal planar geometry around the boron atom. The remaining p orbital remains unhybridized and is involved in π bonding. (Hybridization, sp² hybridization, π bonding)
How Are BF3 Hybrid Orbitals Formed?
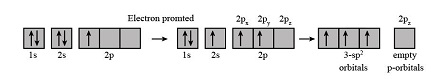
The formation of BF3 hybrid orbitals involves the following steps:
- Step 1: Promotion of Electrons – One electron from boron’s 2s orbital is promoted to the 2p orbital, resulting in three unpaired electrons.
- Step 2: Hybridization – The 2s orbital and two 2p orbitals hybridize to form three sp² hybrid orbitals.
- Step 3: Bond Formation – These sp² hybrid orbitals overlap with the p orbitals of fluorine atoms, forming three sigma bonds.
📌 Note: The unhybridized p orbital does not participate in sigma bonding but is involved in back-bonding with fluorine, contributing to the molecule’s stability. (Sigma bonds, back-bonding, molecular stability)
Molecular Geometry and Bond Angles

The trigonal planar geometry of BF3 is a direct result of sp² hybridization. This geometry ensures that the three sp² hybrid orbitals are oriented at 120° bond angles, minimizing electron repulsion. The absence of lone pairs on the central boron atom further stabilizes this structure. (Trigonal planar geometry, bond angles, electron repulsion)
| Parameter | Value |
|---|---|
| Hybridization | sp² |
| Geometry | Trigonal Planar |
| Bond Angle | 120° |

Applications and Significance of BF3 Hybrid Orbitals

BF3’s hybrid orbitals play a vital role in its applications, such as a catalyst in organic synthesis and as a reagent in industrial processes. Understanding its bonding structure helps chemists predict reactivity and design new compounds. (Catalyst, organic synthesis, industrial processes)
Key Takeaways: BF3 Hybrid Orbitals Checklist

- BF3 exhibits sp² hybridization with three sp² hybrid orbitals.
- The molecule has a trigonal planar geometry with 120° bond angles.
- The unhybridized p orbital is involved in π bonding or back-bonding.
- BF3 is widely used in catalysis and industrial chemistry.
In summary, BF3 hybrid orbitals are a prime example of how hybridization influences molecular structure and bonding. By understanding sp² hybridization, trigonal planar geometry, and the role of unhybridized orbitals, we gain valuable insights into BF3’s properties and applications. Whether for academic study or practical applications, mastering this concept is essential for anyone interested in molecular chemistry. (Molecular structure, hybridization, chemical bonding)
What is the hybridization of BF3?
+
BF3 exhibits sp² hybridization, involving one s orbital and two p orbitals of the boron atom.
Why does BF3 have a trigonal planar geometry?
+
The trigonal planar geometry results from the arrangement of three sp² hybrid orbitals at 120° bond angles, minimizing electron repulsion.
What is the role of the unhybridized p orbital in BF3?
+
The unhybridized p orbital is involved in π bonding or back-bonding with fluorine, contributing to the molecule’s stability.