Chloroform Lewis Dot Structure: A Simple Guide
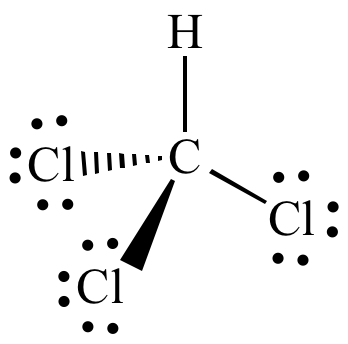
Understanding the Chloroform Lewis Dot Structure is essential for anyone studying chemistry, especially organic chemistry. Chloroform, with the chemical formula CHCl₃, is a common solvent used in laboratories and industries. Its Lewis dot structure provides a clear visualization of the electron distribution and bonding within the molecule. This guide will walk you through the process of drawing the Lewis dot structure for chloroform, ensuring you grasp the fundamentals with ease.
What is a Lewis Dot Structure?
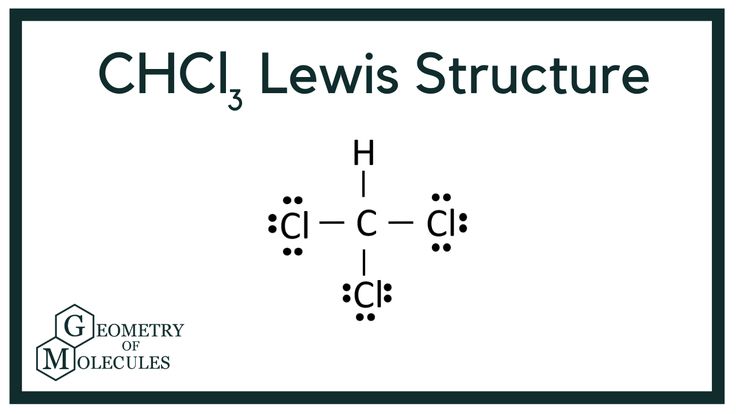
A Lewis dot structure is a diagram that shows the bonding between atoms in a molecule and the lone pairs of electrons that may exist. It uses dots to represent valence electrons and lines to represent covalent bonds. Understanding this structure is crucial for predicting molecular geometry and reactivity.
Steps to Draw the Chloroform Lewis Dot Structure
Step 1: Determine the Central Atom
In chloroform (CHCl₃), carbon © is the central atom because it is less electronegative than chlorine (Cl) and hydrogen (H).
Step 2: Count the Total Valence Electrons
- Carbon © has 4 valence electrons.
- Hydrogen (H) has 1 valence electron.
- Chlorine (Cl) has 7 valence electrons (and there are 3 Cl atoms).
Total valence electrons = 4 © + 1 (H) + 3 × 7 (Cl) = 26 electrons.
Step 3: Connect Atoms with Single Bonds
Place the carbon atom in the center and connect it to the hydrogen and chlorine atoms using single bonds. Each single bond uses 2 electrons, so 4 bonds will use 8 electrons.
Step 4: Complete the Octet for Each Atom
- Hydrogen (H) already has a full outer shell with 2 electrons.
- Chlorine (Cl) needs 8 electrons to complete its octet. Distribute the remaining electrons around the chlorine atoms.
- Carbon © will have a complete octet after forming bonds with H and Cl.
📌 Note: Chlorine atoms will have three pairs of lone electrons each after bonding.
Step 5: Verify the Structure
Ensure all atoms have a complete octet (except hydrogen) and that the total number of valence electrons (26) is accounted for.
Chloroform Lewis Dot Structure: Visual Representation
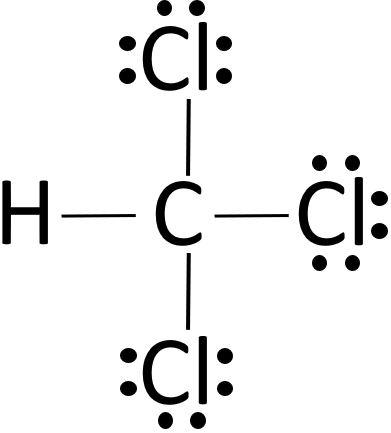
The final structure will show:
- Carbon © in the center.
- One hydrogen (H) atom single-bonded to carbon.
- Three chlorine (Cl) atoms single-bonded to carbon.
- Each chlorine atom will have six lone pair electrons (three pairs).
| Atom | Valence Electrons | Bonds | Lone Pairs |
|---|---|---|---|
| Carbon (C) | 4 | 4 | 0 |
| Hydrogen (H) | 1 | 1 | 0 |
| Chlorine (Cl) | 7 | 1 | 3 |
Key Takeaways

- Carbon is the central atom in chloroform.
- Total valence electrons in CHCl₃ are 26.
- Each chlorine atom has 6 lone pair electrons.
- The structure satisfies the octet rule for all atoms except hydrogen.
Checklist for Drawing Lewis Dot Structures

- Determine the central atom.
- Count total valence electrons.
- Connect atoms with single bonds.
- Complete the octet for each atom.
- Verify the structure.
Chloroform Lewis Dot Structure, Lewis Structure of CHCl3, Drawing Lewis Structures
What is the central atom in chloroform?
+The central atom in chloroform (CHCl₃) is carbon (C).
How many valence electrons are in chloroform?
+Chloroform has a total of 26 valence electrons.
Why does chlorine have lone pairs in chloroform?
+Chlorine has 7 valence electrons and forms one bond, leaving 6 electrons as lone pairs to complete its octet.
By following this simple guide, you’ll master the Chloroform Lewis Dot Structure and gain confidence in drawing Lewis structures for other molecules. Practice makes perfect, so keep exploring and experimenting with different compounds!


