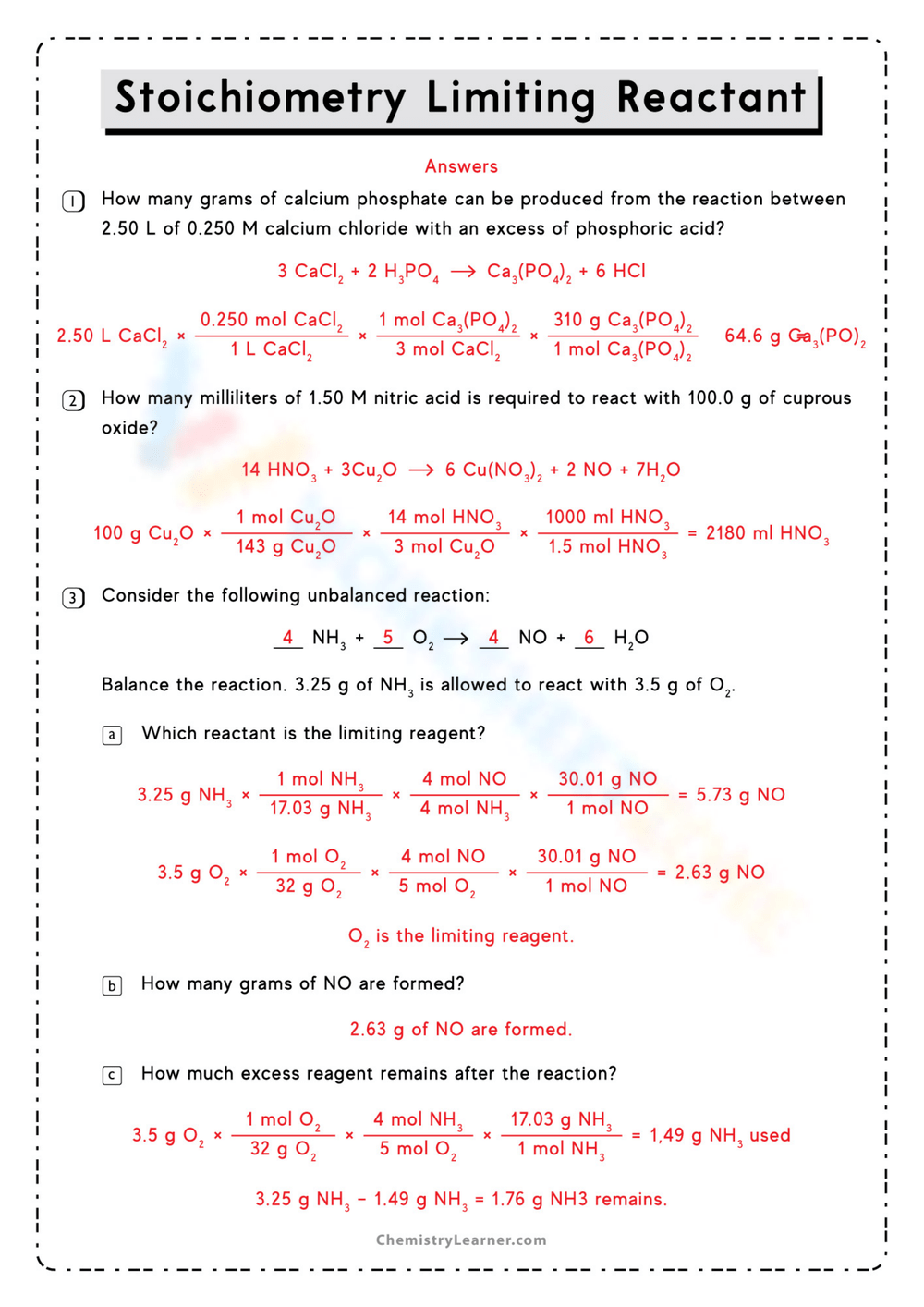
Master Limiting Reactant with Practice Problems Worksheet
<!DOCTYPE html>
Understanding the concept of limiting reactant is crucial in chemistry, as it determines the maximum amount of product that can be formed in a chemical reaction. By mastering this concept, students can accurately predict reaction outcomes and improve their problem-solving skills. A limiting reactant practice problems worksheet is an excellent tool to reinforce this knowledge through hands-on practice.
What is a Limiting Reactant?

In a chemical reaction, the limiting reactant is the reactant that is completely consumed, limiting the amount of product formed. The other reactants are in excess and are not fully used up. Identifying the limiting reactant is essential for calculating theoretical yields and understanding reaction stoichiometry.
Why Use a Practice Problems Worksheet?

A limiting reactant practice problems worksheet provides structured exercises to help students apply theoretical knowledge to practical scenarios. These worksheets often include step-by-step solutions, allowing learners to check their work and identify mistakes. Consistent practice builds confidence and ensures a deeper understanding of the concept.
Key Steps to Solve Limiting Reactant Problems

Solving limiting reactant problems involves a systematic approach. Here’s a breakdown of the steps:
- Balance the Chemical Equation: Ensure the equation is balanced to reflect accurate mole ratios.
- Convert Masses to Moles: Use molar masses to convert given masses of reactants into moles.
- Determine the Limiting Reactant: Compare the mole ratio of the reactants to the balanced equation.
- Calculate the Product: Use the limiting reactant to find the maximum amount of product formed.
📌 Note: Always double-check your units and calculations to avoid errors.
Benefits of Mastering Limiting Reactant

Mastering limiting reactant concepts offers several advantages:
- Improved Exam Performance: Chemistry exams often include stoichiometry problems, and mastering limiting reactants is key to scoring well.
- Real-World Applications: Understanding limiting reactants is vital in industries like pharmaceuticals and manufacturing, where precise product yields are critical.
- Enhanced Problem-Solving Skills: Regular practice sharpens analytical thinking and logical reasoning abilities.
Sample Worksheet Problem

Here’s an example problem to illustrate the process:
| Reactant | Mass (g) | Molar Mass (g/mol) |
|---|---|---|
| Hydrogen (H₂) | 4 | 2 |
| Oxygen (O₂) | 32 | 32 |

For the reaction: 2H₂ + O₂ → 2H₂O, determine the limiting reactant and calculate the mass of water produced.
📌 Note: This problem requires converting masses to moles and comparing them to the balanced equation.
To wrap up, a limiting reactant practice problems worksheet is an invaluable resource for mastering stoichiometry. By following a structured approach and practicing regularly, students can confidently tackle complex problems and excel in chemistry. (limiting reactant problems, stoichiometry practice, chemistry worksheets)
What is the limiting reactant in a chemical reaction?
+The limiting reactant is the reactant that is completely consumed in a reaction, limiting the amount of product formed.
How do I identify the limiting reactant?
+Convert the masses of reactants to moles and compare their mole ratios to the balanced chemical equation.
Why is practicing limiting reactant problems important?
+Practicing these problems improves problem-solving skills, exam performance, and understanding of real-world chemistry applications.



