Molar Mass of KOH: Quick Guide & Calculation Tips

Understanding the molar mass of KOH is essential for anyone working in chemistry, whether you’re a student, researcher, or industry professional. Potassium hydroxide (KOH) is a versatile compound used in various applications, from soap making to battery production. Knowing its molar mass is crucial for accurate calculations in chemical reactions and experiments. In this guide, we’ll walk you through the KOH molar mass calculation, provide tips for precision, and answer common questions to ensure you’re well-equipped with the knowledge you need.
What is the Molar Mass of KOH?

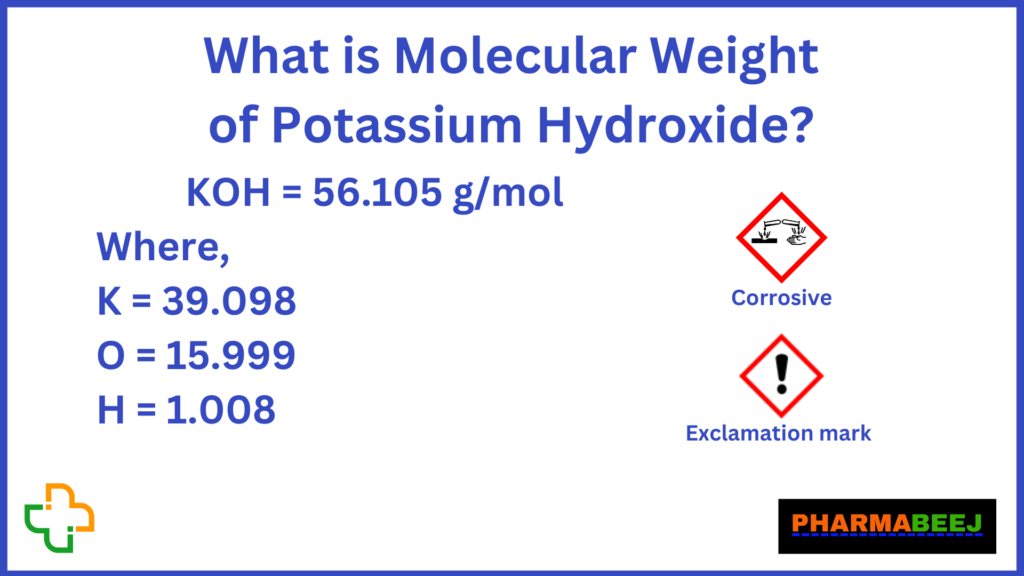
The molar mass of KOH is the sum of the atomic masses of its constituent elements: potassium (K), oxygen (O), and hydrogen (H). Using the periodic table, we find the atomic masses as follows:
- Potassium (K): 39.10 g/mol
- Oxygen (O): 16.00 g/mol
- Hydrogen (H): 1.01 g/mol
By adding these values, the molar mass of KOH is calculated as:
39.10 + 16.00 + 1.01 = 56.11 g/mol.
📌 Note: Always ensure you use the most accurate atomic masses from a reliable periodic table for precise calculations.
Step-by-Step Calculation of KOH Molar Mass

To calculate the molar mass of KOH, follow these simple steps:
1. Identify the elements: KOH consists of potassium (K), oxygen (O), and hydrogen (H).
2. Find atomic masses: Look up the atomic masses of K, O, and H from the periodic table.
3. Sum the masses: Add the atomic masses together to get the molar mass of KOH.
Here’s a quick table for reference:
| Element | Atomic Mass (g/mol) |
|---|---|
| Potassium (K) | 39.10 |
| Oxygen (O) | 16.00 |
| Hydrogen (H) | 1.01 |
| Total (KOH) | 56.11 |

Calculation Tips for Accuracy

When determining the molar mass of KOH, keep these tips in mind:
- Use precise atomic masses: Small errors in atomic masses can lead to significant discrepancies in molar mass calculations.
- Double-check your math: Ensure you’ve added the atomic masses correctly.
- Consider the state of KOH: While the molar mass remains the same, the state (solid, liquid, or solution) may affect its application in experiments.
💡 Note: For commercial applications, consult a KOH molar mass calculator or chemical supplier for verified data.
Practical Applications of KOH Molar Mass
Knowing the molar mass of KOH is vital in:
- Chemical reactions: Accurate stoichiometry ensures successful reactions.
- Solution preparation: Essential for creating solutions with specific concentrations.
- Industrial processes: Used in manufacturing soaps, batteries, and more.
Checklist for Calculating KOH Molar Mass
Before you start, ensure you have:
- A reliable periodic table.
- A calculator for precise addition.
- Clear understanding of KOH’s chemical formula (KOH).
To recap, the molar mass of KOH is 56.11 g/mol, calculated by summing the atomic masses of potassium, oxygen, and hydrogen. Mastering this calculation is key for both academic and industrial chemistry tasks.
What is the molar mass of KOH?
+The molar mass of KOH is 56.11 g/mol, calculated by adding the atomic masses of potassium (39.10 g/mol), oxygen (16.00 g/mol), and hydrogen (1.01 g/mol).
Why is knowing the molar mass of KOH important?
+Knowing the molar mass of KOH is crucial for accurate chemical calculations, solution preparation, and industrial applications like soap making and battery production.
Can the molar mass of KOH change?
+No, the molar mass of KOH remains constant at 56.11 g/mol, regardless of its physical state (solid, liquid, or solution).
Related Keywords: molar mass of KOH, KOH molar mass calculation, KOH molar mass calculator, potassium hydroxide molar mass, chemical calculations, periodic table, atomic masses.



