Molar Mass of C2H2: Quick Calculation Guide

Understanding the molar mass of C2H2 is essential for students and professionals in chemistry, as it forms the basis for many calculations in stoichiometry and chemical reactions. Acetylene (C2H2) is a crucial compound in various industries, including welding and chemical synthesis. This guide provides a quick and easy method to calculate the molar mass of C2H2, ensuring accuracy and efficiency in your work. Whether you're studying for an exam or working in a lab, mastering this calculation is invaluable.
What is Molar Mass and Why is it Important?
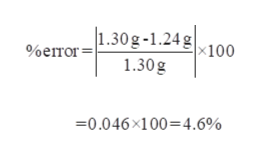
Molar mass represents the mass of one mole of a substance, expressed in grams per mole (g/mol). It is a fundamental concept in chemistry, used to determine the amount of a substance in a reaction or solution. For C2H2 (acetylene), knowing its molar mass is critical for applications like gas stoichiometry, chemical synthesis, and industrial processes. Molar mass calculations rely on the atomic masses of constituent elements, making it a cornerstone of chemical analysis.
Step-by-Step Guide to Calculate the Molar Mass of C2H2

Step 1: Identify the Elements in C2H2
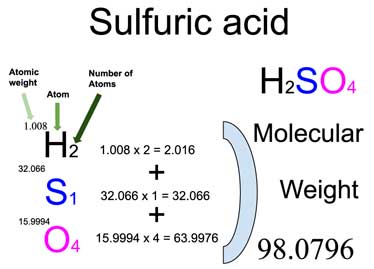
C2H2 consists of carbon © and hydrogen (H). Each element’s atomic mass is necessary for the calculation. Refer to the periodic table for accurate values: Carbon © ≈ 12.01 g/mol and Hydrogen (H) ≈ 1.01 g/mol.
Step 2: Determine the Number of Atoms
From the formula C2H2, there are 2 carbon atoms and 2 hydrogen atoms. Multiply the atomic mass of each element by its respective number of atoms.
Step 3: Calculate the Total Molar Mass
Use the formula:
Molar Mass of C2H2 = (Atomic Mass of C × Number of C atoms) + (Atomic Mass of H × Number of H atoms)
Substitute the values:
Molar Mass of C2H2 = (12.01 g/mol × 2) + (1.01 g/mol × 2) = 24.02 g/mol + 2.02 g/mol = 26.04 g/mol.
| Element | Atomic Mass (g/mol) | Number of Atoms in C2H2 |
|---|---|---|
| Carbon (C) | 12.01 | 2 |
| Hydrogen (H) | 1.01 | 2 |
✨ Note: Always use the most accurate atomic masses from a reliable periodic table for precise calculations.
Calculating the molar mass of C2H2 is straightforward once you understand the process. By identifying the elements, determining the number of atoms, and using their atomic masses, you can accurately find the molar mass of acetylene. This skill is essential for various chemical applications, ensuring you can confidently handle stoichiometry problems and lab calculations. Master this technique to enhance your proficiency in chemistry, whether for academic or professional purposes.
What is the molar mass of C2H2?
+The molar mass of C2H2 (acetylene) is approximately 26.04 g/mol.
Why is molar mass important in chemistry?
+Molar mass is crucial for stoichiometry, determining reaction yields, and understanding the composition of substances in chemical processes.
How do I find atomic masses for molar mass calculations?
+Refer to a periodic table for accurate atomic masses of elements like carbon and hydrogen.
molar mass calculation,acetylene formula,chemical stoichiometry,periodic table,atomic mass,chemistry basics,C2H2 applications,gas calculations,laboratory techniques,chemical reactions.