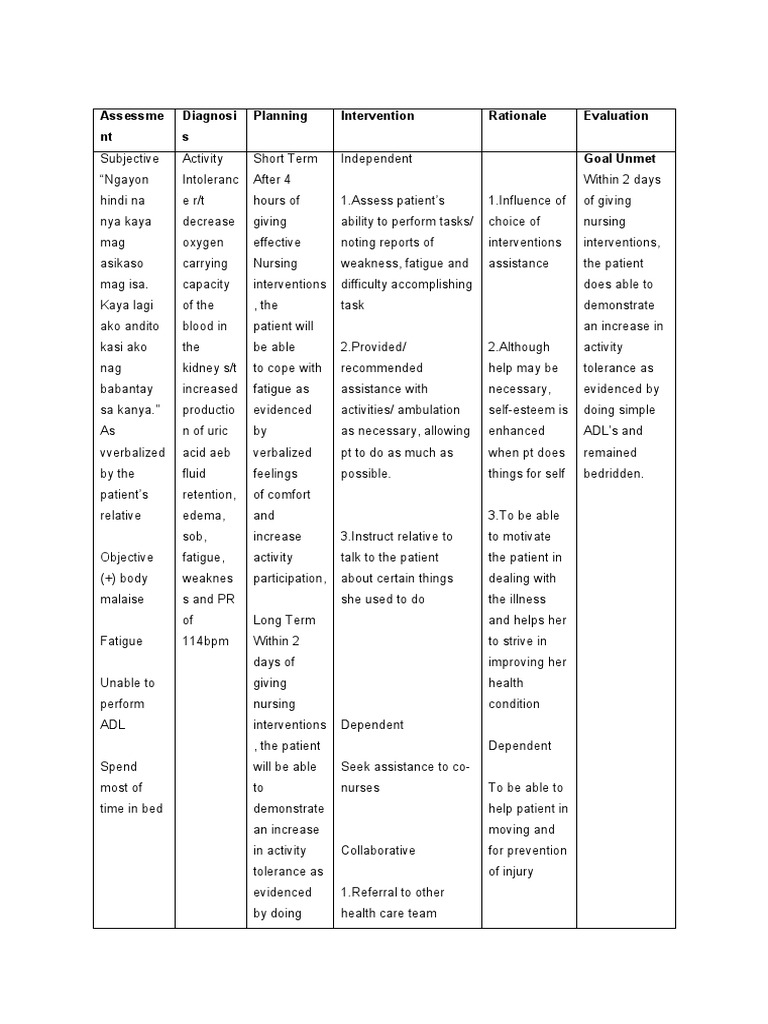
How Many Electrons Does Carbon Have?

Carbon is one of the most fundamental elements in chemistry, playing a crucial role in organic compounds and life itself. A common question that arises is, “How many electrons does carbon have?” Understanding the electron configuration of carbon is essential for students, researchers, and anyone interested in chemistry or materials science. In this post, we’ll explore the answer to this question, delve into carbon’s electron structure, and provide practical insights for both informational and commercial purposes.
How Many Electrons Does Carbon Have?
Carbon, with the atomic number 6, has 6 electrons in its neutral state. These electrons are arranged in specific energy levels or shells around the nucleus. The electron configuration of carbon is 1s² 2s² 2p², meaning it has 2 electrons in the first shell and 4 electrons in the second shell.
| Shell | Electrons |
|---|---|
| First (K) | 2 |
| Second (L) | 4 |

This configuration explains carbon’s ability to form four covalent bonds, making it a key player in organic chemistry and materials like graphene and diamonds.
Why Does Carbon’s Electron Configuration Matter?

Carbon’s electron arrangement is vital for its chemical behavior. With 4 electrons in the outermost shell, carbon can easily share electrons to form stable bonds with other atoms. This property is why carbon is the backbone of organic molecules, including DNA, proteins, and fuels.
For commercial-intent visitors, understanding carbon’s electrons is crucial in industries like nanotechnology, pharmaceuticals, and energy storage. Products like carbon fiber, activated carbon, and carbon-based batteries rely on this knowledge.
💡 Note: Carbon’s unique electron configuration allows it to form millions of compounds, making it indispensable in both nature and industry.
Key Takeaways: Carbon’s Electrons

- Atomic Number: 6
- Total Electrons: 6
- Electron Configuration: 1s² 2s² 2p²
- Valence Electrons: 4
For informational-intent audiences, this knowledge is foundational for studying chemistry, biology, and materials science. For commercial-intent visitors, it’s essential for developing carbon-based products and technologies.
Practical Applications of Carbon’s Electrons

- Organic Chemistry: Carbon’s ability to form multiple bonds enables the creation of complex molecules.
- Nanotechnology: Graphene and carbon nanotubes leverage carbon’s electron structure for advanced materials.
- Energy Storage: Carbon is used in batteries and supercapacitors due to its electron mobility.
📌 Note: Carbon’s versatility stems from its electron configuration, making it a cornerstone of modern technology.
In summary, carbon has 6 electrons, arranged in a configuration that allows it to form four bonds. This property is fundamental to its role in chemistry, biology, and industry. Whether you’re a student, researcher, or entrepreneur, understanding carbon’s electrons is key to unlocking its potential.
How many electrons does carbon have in its outer shell?
+Carbon has 4 electrons in its outer shell (valence electrons), allowing it to form four covalent bonds.
What is the electron configuration of carbon?
+Carbon’s electron configuration is 1s² 2s² 2p², with 2 electrons in the first shell and 4 in the second.
Why is carbon’s electron configuration important?
+It determines carbon’s ability to form bonds, making it essential for organic compounds and advanced materials.
Related Keywords: electron configuration of carbon, carbon valence electrons, carbon atomic structure, carbon in organic chemistry, carbon-based materials.